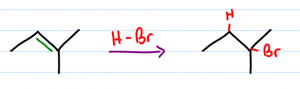Reaction Overview: The hydrohalogenation of alkenes involves breaking a carbon to carbon double bond, followed by the electrophilic addition of a hydrogen atom and halogen. The halide will add to the more substituted carbon following Markovnikov's rule. The product is a haloalkane also called an alkyl halide.
Summary of Hydrohalogenation Mechanism
- Nucleophilic pi bond reaches for electrophilic H in H-X, pi bond breaks in the process
- H adds to the less substituted carbon atom following Markovnikov's rule
- More substituted carbon is now deficient getting a formal charge of +1
- Negative halide in solution attacks the carbocation forming a bond
- Product is a haloalkane – also known as alkyl halide
Mechanism Overview and Explanation
(watch my Hydrohalogenation video to see the detailed step-by-step mechanism in action)
Key Reaction Notes:
- This reaction has a carbocation intermediate and therefore follows Markovnikov's rule
- Look out for carbocation rearrangements
- This reaction must be carried out in an ‘inert' solvent
- This reaction is regioselective – halide adds to more substituted carbon
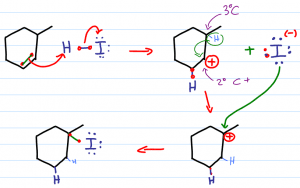
Mechanism for hydrohalogenation with a hydride-shift (carbocation rearrangement)
What's Really Going On In This Reaction?
Hydrohalogenation, an electrophilic alkene addition reaction, is highly useful as a precursor reaction in multi-step organic chemistry synthesis.
Understanding the Molecules:
H-X molecules such as H-I, H-Br and H-Cl are highly polar molecules. The halogen is highly electronegative and will ‘hog' the electrons between itself and hydrogen. This concentration of electrons on the halogen makes it partially negative, while pulling the electrons away from hydrogen makes H partially positive.
(You will seldom see this reaction for H-F due to fluorine's high electronegativity and poor reactivity)
Unlike sigma (single) bonding electrons, the pi bond sits very high and very low on the carbon skeleton. This allows it to be easily ‘distracted' by other nearby molecules. These electrons are highly nucleophilic (attracted to positive) and will reach out for a passing electrophile (positive or partially positive).
Breaking The Pi Bond:
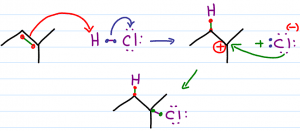
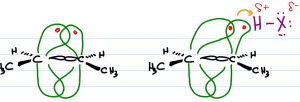 When an H-X bond such as H-Cl or H-Br gets close to the alkene, the pi bonds will reach out to grab the partially positive hydrogen atom. In order to form this bond, one of the pi electrons must let go of the carbon atom that it's bound to.
When an H-X bond such as H-Cl or H-Br gets close to the alkene, the pi bonds will reach out to grab the partially positive hydrogen atom. In order to form this bond, one of the pi electrons must let go of the carbon atom that it's bound to.
In deciding which carbon atom we must follow Markovnikov's rule and ask ourselves which pi bond is more capable of holding a positive charge. The more substituted the carbon atom, the more stable the resulting carbocation.
The other carbon atom hasn't let go of its pi electrons and is now singly bound to the hydrogen atom. This is how the less substituted carbon atom winds up bound to hydrogen.
The hydrogen atom is capable of forming just a single bond. As it forms a bond with carbon it must let go of the halogen. This allows the halogen to grab the electrons that used to bind it to hydrogen, and float away in solution with a complete octet and negative charge.
Attack of the Halide:
Negative halogens are nucleophiles. Nucleophiles are attracted to electrophiles (positive charges).
The lone halogen will use one of it's lone electron pairs to attack the carbocation forming a sigma bond. Since the carbocation was on the more substituted carbon, the halogen winds up attached at the more substituted carbon as well.
Purpose Of Inert Solvent:
The choice of solvent will make a big difference in the overall reaction. Think of your inert solvents as ‘I don't care' solvents. They serve the single purpose of dissolving the reactants and reagents, but they don't interfere in the actual process. Inert solvents for this reaction include CH2Cl2, CCl4 and more. Notice that they are NOT polar protic.
The use of a non-inert solvent or polar protic solvent like water will result in a very different reaction. At the point where the halide breaks away from hydrogen, the solvent ‘butts in'. The polar protic solvent will use its partially positive hydrogen atoms to surround or ‘cage' the negative halogen. This prevents the halogen from attacking the carbocation. Instead, one of the polar protic solvent molecules will use a lone pair of electrons to attack the carbocation.
For example, if carried out in water, the final product will be an alcohol. If carried out in alcohol the product will be an ether.
See the hydrohalogenation reaction come to life along with a few practice examples in my alkene halogenation video: