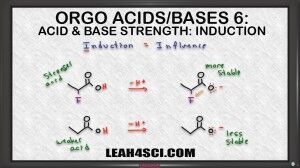
 The inductive effect is likely the most tricky when it comes to ranking acids and bases in organic chemistry. In this video I show you how to view atoms as ‘cheerleaders'. This will help you get a better understanding of the concept and help you rank acids and bases.
The inductive effect is likely the most tricky when it comes to ranking acids and bases in organic chemistry. In this video I show you how to view atoms as ‘cheerleaders'. This will help you get a better understanding of the concept and help you rank acids and bases.
(click HERE to watch this video on YouTube or catch video transcript here)
Watch Previous Video: R = Resonance
Watch Next Video: O = Orbital Hybridization
Ready to test your Acid/Base skills? Try my FREE acid/base practice quiz


