 Ortho, Meta and Para refer to the relationship between substituents on a disubstituted benzene ring. In the context of Electrophilic Aromatic Substitution, understanding the chemistry of substituents will help you figure out where to direct the incoming electrophile on a substituted benzene ring.
Ortho, Meta and Para refer to the relationship between substituents on a disubstituted benzene ring. In the context of Electrophilic Aromatic Substitution, understanding the chemistry of substituents will help you figure out where to direct the incoming electrophile on a substituted benzene ring.
Ortho Meta Para Definitions
Let’s backtrack all the way to nomenclature from your first (second?) chapter in orgo 1. In IUPAC naming, every substituent is designated with a number.
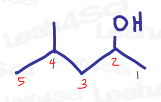
Given a 5 carbon chain with a methyl on carbon 4 and alcohol on carbon 2, you get 4-methyl-2-pentanol.
But that wasn’t the only way to indicate the relationships of substituents, was it? Think back to cis and trans or to E and Z.
When it comes to a disubstituted benzene, using the terms ‘ortho, meta and para’ is simply another way to indicate the relationship of the substituents to each other, rather than their overall placement on the ring.
For example, 1,2-dimethylbenzene tells us we have methyl groups both on carbons 1 and 2.
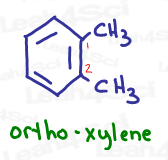
Ortho-dimethylbenzene (ortho-xylene or o-xylene) tells us that the 2 groups are directly next to each other on the ring.
Common Mistake Warning: Despite their similar appearance, these relationships ONLY apply to benzene rings and NOT cyclohexane rings.
Ortho
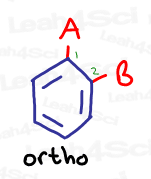
Ortho represents a 1,2 relationship, or 2 groups that are directly near each other on the benzene ring.
Meta
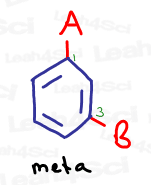
Meta represents a 1,3 relationship, or groups that are separated by one carbon on the benzene ring.
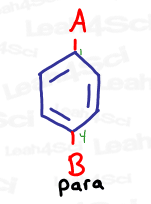
Para
Para represents a 1,4 relationship, or groups that are opposite each other on the benzene ring.
Use the friendly OMP monster (explained in the video below) to help you quickly recognize these relationships.
Ok, so we have fancy names for these relationships. Now what?
When you first learned EAS reactions, you focused on adding a simple super-electrophile to benzene. Use these videos to review EAS reactions.
But organic chemistry is never as simple as what you learn in lecture, is it?After mastering the simple reactions, you’ll be asked to complete EAS reactions on more complicated and substituted aromatic compounds.
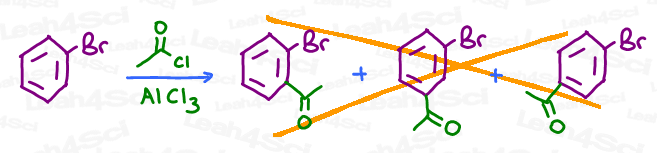
For example, say you’re asked to complete a Friedel-Crafts Acylation on a bromobenzene. Do you add it to the ortho, meta, or para position? Or, do you simply show all 3 options for triple credit?
Not quite!
WHERE you place the incoming electrophile is directly influenced by the chemical nature of the substituent(s) already on the benzene ring. Initial substituents act as directors for the incoming groups.
Important Vocabulary for OMP Directing Effects
Let’s first understand the language before jumping into the why and how.
Directing / Director / Directing Effect
A group already on benzene that will dictate where the incoming electrophile will be added.
Activating Group
A group already on benzene that activates the ring towards Electrophilic Aromatic Substitution reactions. A group that will speed up the EAS reaction.
Deactivating Group
A group already on benzene that deactivates the ring towards Electrophilic Aromatic Substitution reactions. A group that will slow down (and sometimes completely halt) the EAS reaction.
Electron Donating Groups – EDG
A group already on benzene that puts negativity into the ring, either directly through resonance or indirectly through induction.
Electron Withdrawing Groups – EWG
A group already on benzene that pulls negativity out of the ring, either directly through resonance or indirectly through induction.
Now that we can speak gibberish, let’s translate this to some practical applications.
Simply put, electron donating groups are activators and therefore act as ortho and para directors. Electron withdrawing groups are deactivators and therefore act as meta directors. (Halogens are the annoying exception.)
On my EAS Cheat Sheet, we look at the most common substituents and rank them from strong to weak activators and deactivators.
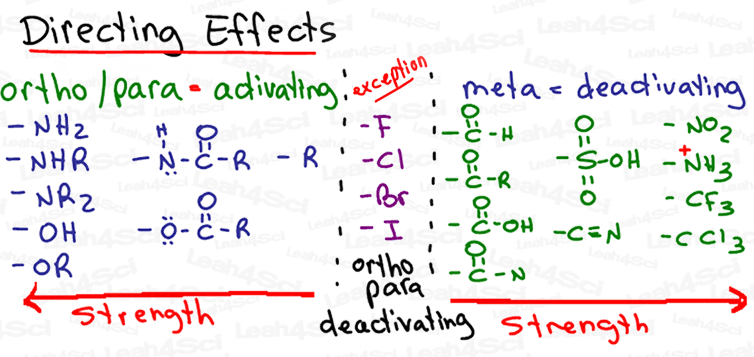
And this is where most students will start to studiously memorize the list, hoping to make sense of the information, but they ultimately freak out on exam day.
But not you! Because we’re about to break this down to the point where you will be able to evaluate any substituent and immediately and logically predict its EAS directing effects.
First, a quick review of the Electrophilic Aromatic Substitution Mechanism:
- One pi bond in benzene breaks open to attack a super (positively charged) electrophile.
- The attacking carbon is now bound to the electrophile, while the other former pi-bound carbon is now positively charged due to having lost its fourth bond.
- Sigma-complex resonance helps stabilize the intermediate.
- A weak base grabs hydrogen off the newly substituted carbon atom, collapsing its electrons towards the carbocation.
- This reforms a pi bond between the 2 carbons, thus re-establishing resonance and aromaticity.
Note: E+ represents the super-electrophile, and the attached E represents the electrophile having attached to the benzene ring.
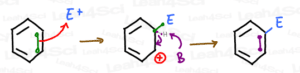
To fully understand the directing effects, we focus on the intermediate of this reaction rather than the O/M/P final products.
Which intermediate? The positively charged sigma-complex.
Review these tutorials first if you’re not comfortable with carbocation stability or resonance.
Sigma-Complex Resonance
Go back to the positively charged intermediate above. Notice how the carbocation is capable of not one, but TWO additional resonance forms?
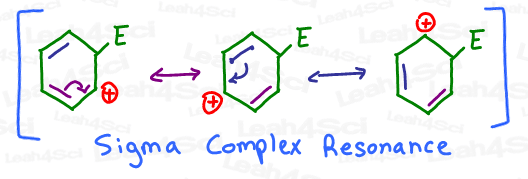
Now think back to orgo basics.
What increases the likelihood of a reaction taking place?
Stability of the intermediate!
The more stable the intermediate, the more likely that intermediate will form.
The less stable the intermediate… well, molecules just like people, want to avoid pain. And if forced to react, they will stall and grumble every step of the way, thus slowing down the reaction.
First, notice the position of the carbocation in the sigma-complex intermediate.
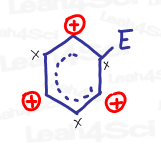
See how it has 3 distinct locations? Try as you might, you cannot get the carbocation onto any other position.
This is key!
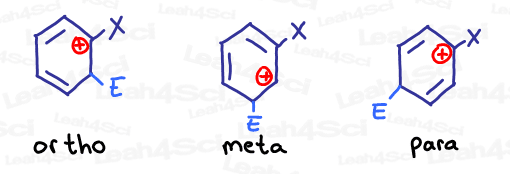
Pay attention to the pattern. Let’s say we add a new electrophile to an already substituted benzene ring. What 3 positions can hold the carbocation in each ortho, meta and para addition?
Note: X represents the substituent already present on the ring, and E represents the newly added electrophile.
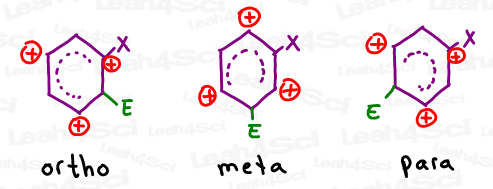
Notice how ortho and para have the same carbocation intermediate patterns, while meta is the exact opposite?
In the absence of additional resonance, we care most about the intermediate with a carbocation closest to the substituted carbon.
Which sigma-complex do you think is most stable? The one with an electron donating, or electron withdrawing group?
If you said the carbonyl is less stable, you are correct. The partially positive carbonyl carbon (EWG) would be pissed to have a carbocation nearby.
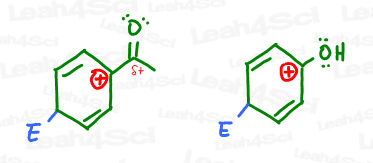 On the other hand, not only is phenol’s oxygen stable near the carbocation, it can even donate its electrons INTO the ring to further stabilize the positive sigma-complex intermediate.
On the other hand, not only is phenol’s oxygen stable near the carbocation, it can even donate its electrons INTO the ring to further stabilize the positive sigma-complex intermediate.
The video below explains and demonstrates this in more detail – complete with step-by-step resonance structures.
Activating Groups as Ortho/Para Directors
Instead of memorization, recognize activating groups as substituents that bring negativity (in a good way) towards the ring. This can be in the form of a lone pair or very electronegative groups.
Since these negative groups are happy to sit directly near a carbocation, and since the carbocation forms AT their position only in ortho and para addition, activating groups force incoming electrophiles to add to the ortho or para position.
This makes EDGs ortho and para directors.
Different reactions will have different percentages for ortho and para positions. This has to be evaluated in a lab setting and so you will NOT be expected to differentiate. The one exception is to know that a bulky substituent will force para addition simply due to the steric hindrance of its bulk.
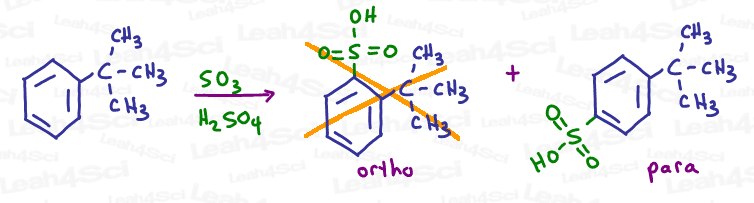
Deactivating Groups as Meta Directors
Electron withdrawing groups like to pull negativity away from the ring. This makes a positive intermediate very unstable, as it desires negativity rather than positivity to stabilize it.
As such, electron withdrawing groups slow down, thus deactivating, the EAS reaction. And when they finally agree to react (slowly), these groups want to be as far away from the positive charge as possible.
A quick review of sigma-complex resonance shows that the meta addition pattern allows the carbocation to skip the substituent carbon.
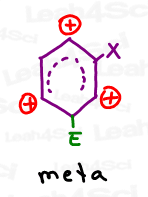
And while not the most stable, this is the lesser of 2 evils and thus the preferred pattern.
This is why deactivating electron withdrawing groups act as meta directors.
Halogens are the single exception to this trend.
Yes, they are electron donating groups, but they do it so begrudgingly that they intentionally slow down the reaction. This makes the halogen a deactivator, but still an ortho/para director.
In Summary
Ortho, Meta and Para refer to the 1-2, 1-3, and 1-4 relationships between benzene substituents. In Electrophilic Aromatic Substitution reactions, O/M/P directing effects help us figure out where to place the incoming electrophile. Electron Donating Groups activate the ring for ortho and para addition. Electron Withdrawing Groups deactivate the ring for meta addition. Halogens are the one exception.
I want to hear from you!
Do you now feel ready to conquer EAS practice questions WITHOUT relying on memorized substituents?