When you hear n-butyl, secbutyl, isobutyl and tert butyl.
Do these terms scare you?
Learning Organic Chemistry is like learning a new language where certain prefixes and suffixes will mean different things. Different ways of arranging bits and pieces will bring you to different variations of compounds. But just like a new language, the more you see it, hear it, and practice it, the better you become.
 When it comes to naming branched organic compounds, following the IUPAC nomenclature rules quickly becomes overwhelming.
When it comes to naming branched organic compounds, following the IUPAC nomenclature rules quickly becomes overwhelming.
More carbon atoms added in a branched compound = more rules, and that can easily add up
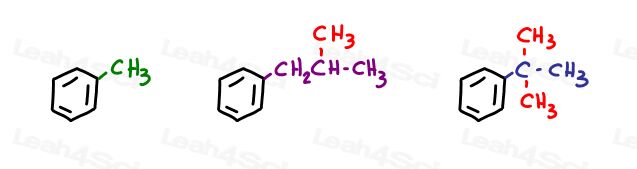
For example, a methyl attached to a propyl substituent,
Or 2 methyl groups attached to an ethyl substituent.
Luckily, some of the small branches are so common, scientist folk before us have created simple alternative names which are allowed in IUPAC nomenclature.
Butyl substituents are most common, but in this tutorial we’ll take a look at propyl and pentyl as well.
Understanding branched substituents
Branched substituents are simply alkyl groups attached to the parent chains.
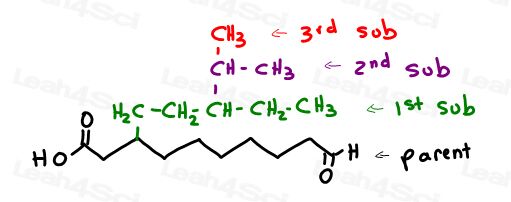
These alkyl substituents have ADDITIONAL alkyl groups coming out of them-
a branch on the branch.
Confused yet?
Instead of worrying about naming compounds branch on branch, (for example: 1-methylethyl), we will picture the linear equivalent of this molecule and name it accordingly.
Need guidance on Primary, Secondary, and Tertiary carbons? See my Pencil Trick Here!
Linear vs. branched substituents
Constitutional Isomers
Recall that constitutional isomers are isomers that differ in their constitution.
Isomers = same molecular formula (same number of each type of atom) but something about them is different.
I think of the connectivity as the constitution, it’s how the molecule is constructed.
If I have 3 carbons in a row, or 2 carbons coming off the first, they differ in their constitution, and that makes them different.
IUPAC naming for branched isomers can get messy as broken down in the video below
To name the branched version, we’ll simply identify the linear constitutional isomer and apply a related name to the branch.
We’ve looked at individual carbons, but for the remainder of this tutorial I’ll be using skeletal structure or line structure.
Not confident with it? See the Drawing Skeletal Structures video below:
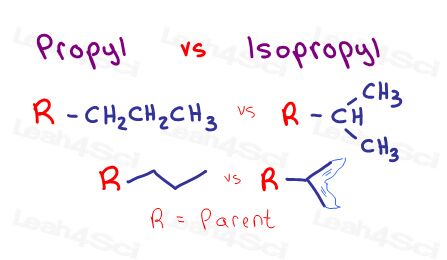
Propyl vs. Isopropyl Substituents
 A 3-carbon substituent is called a propyl group.
A 3-carbon substituent is called a propyl group.
When all 3 carbons are connected in a row you get an n-propyl or ‘normal’ propyl substituent.
However, when that same 3-carbon substituent has one carbon attached to the parent by the second carbon, it gives us an isomer of propyl.
ISOmer of propyl = isopropyl.
Since I enjoy sculpting fantasy creatures, I envision the 3-carbon branch as a mermaid’s tail. I call it the iso tail. You’ll see this same iso-tail in the butyl substituents below.
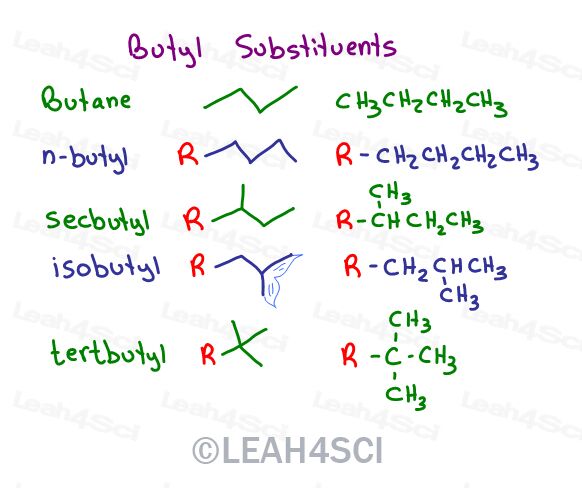
Secbutyl, Isobutyl, and Tert butyl substituents
The prefix ‘but’ tells us there are 4 carbon atoms.
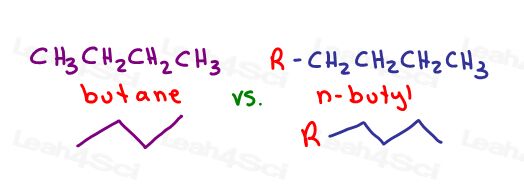
A linear 4-carbon substituent is called butyl or n-butyl.
Given that butyl has more carbon atoms than propyl, we have more options for types of constitutional isomers.
In fact, we have a total of 4 options for butyl substituents.
n-butyl
N-butyl is ‘normal’ butyl and has 4 carbons in a row.
For example, para-butylbenzoic acid
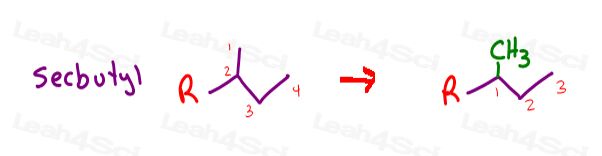
sec-butyl
The least obvious branch doesn’t look like a branch at first.
These substituents appear to have 4 carbons in a row.
The only difference is that instead of attaching the parent chain to carbon 1,
it’s attached at the ‘SEC’ or second carbon.
This is the secondary carbon of the substituent giving us a secondary butyl substituent.
Want to know more about Classifying Carbons? See my Pencil Trick Tutorial Here
That’s how I remember the prefix for sec butyl.
However, if you think of the carbon attached to the parent as carbon #1, look for the longest chain, that’s a propyl NOT a butyl.
This 3-carbon propyl substituent has another 1-carbon methyl substituent coming off of the first carbon.
Isn’t sec-butyl so much easier to say than 1-methylpropyl?
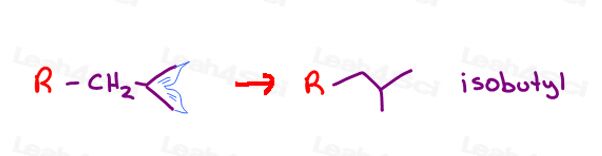
Isobutyl
Remember that mermaids tail for the isopropyl substituent?
If I take sec-butyl and move the methyl from carbon 1 to carbon 2, I can once again envision that fish-tail or mermaids tail.
This 4-carbon iso-tail substituent is isobutyl. Much simpler than 2-methylpropyl.
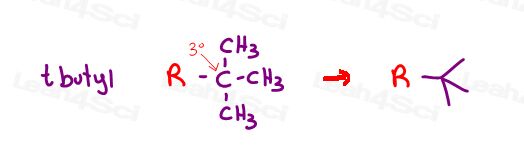
Tert butyl or t-butyl
Finally, if we take the butyl substituent and break the parent so that it’s just 2 instead of 3 carbons long, we now have TWO MORE carbon atoms to attach as two separate methyl groups.
The annoying name for this substituent would be 1,1-dimethylethyl.
Or, if you notice that central carbon #1 is a tertiary carbon (discount the parent chain bond), you can call it tertiary or tert butyl.
You’ll also see it abbreviated as t-butyl.
To summarize the butyls,
A butyl substituent can exist as 4-different constitutional isomers:
N-butyl or normal butyl = linear
Sec butyl, short for 1-methylpropyl
Isobutyl, short for 2-methylpropyl
Tert butyl, short for 1,1-dimethylethyl
A word about pentyl substituents
The prefix ‘pent’ tells us that we have 5 carbons in our chain.
Increasing the number of carbons increases the number of possible substituents.
In fact, here’s what a few minutes of doodling got me:
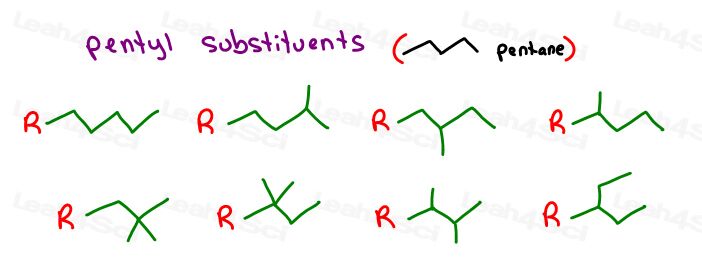
Do you have to memorize special names for all 8 substituents?
A very lucky…NO!
*deep sigh of relief*
In fact, since these structures are not that common, your professor may not even require memorizing ANY special pentyl substituent names.
If you DO have to know them, here are the top 2 to memorize:
N-pentyl or ‘normal’ pentyl, which is just 5 carbons in a row.
And neopoentyl, which looks like a tertbutyl group with an extra CH2 between the t-but group and the parent chain 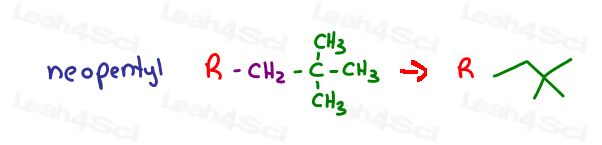
What About Longer Substituents?
Luckily, the shortcuts stop here. As an organic chemistry student you will not have to memorize special names for branched substituents containing more than 5 carbon atoms.
So we have their names, now what?
How does this connect to everything else you’ll cover in organic chemistry?
When starting Orgo 1, this is most important for your naming exams.
Make sure you know:
- How to count these substituents
- How to name them
- And more importantly, how to quickly recognize them in Lewis structure, skeletal structure, or any convoluted shortcuts your professor dreams up.
But these concepts don’t go away after your first nomenclature exam!
While meant to serve as shortcuts in substituent naming only, these common names have been unofficially adapted to naming compounds incorrectly.
I say ‘incorrect’ because they don’t follow IUPAC rules, but are common and accepted enough for your to know them.
For example, halogens are substituents, yet if we turn the halide into the parent, the alkyl chain becomes the substituent.
Bromoethane becomes ethyl bromide.
Can you see how 2-bromopropane can be viewed as isopropyl bromide?

What about alcohol?
If 1-propanol is propyl alcohol,
Have you heard of 2-propanol vs. isopropyl alcohol?
Ask any non-organic chemistry friends and they’ll confirm they’ve heard of the latter.
Ask them about 2-propanol and they’ll look at you with confusion.
If we look at 2-methyl-2-propanol (also 2-methylpropane-2-ol),
Is it so hard to envision this as tertbutyl alcohol?
If you’ve studied your Substitution and Elimination reactions you’ll recognize the de-protonated version, tert butyl oxide or tert butoxide, as the Big Bulky Base that does NOT follow Zaitsev’s rule in E2 reactions.

To get even more proficient with nomenclature I recommend studying the following tutorials:
- Organic Chemistry Functional Groups (Video + Cheat Sheet)
- Naming Organic Compounds (20+ Video series + practice quiz coming soon)
In Conclusion:
Naming branched substituents can get confusing really fast.
Luckily, you can simplify the common branched substituents by memorizing the following shortcuts:
Isopropyl for a branched 3-chain substituent.
Secbutyl, Isobutyl, and tertbutyl for branched 4-carbon substituents.
Neopentyl is the only one to memorize for branched 5-carbon substituents.
I’d love to hear from you.
What are some crazy naming examples that your professor likes to test or discuss?
Let me know in the comments below.


