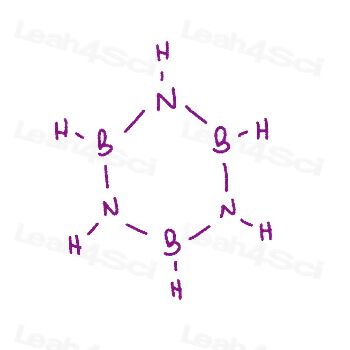
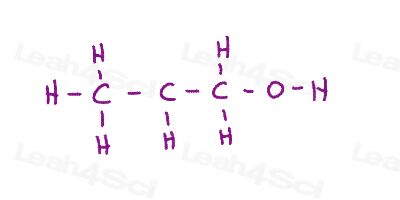Not sure if you've mastered resonance structures? Looking for extra practice?
Not sure if you've mastered resonance structures? Looking for extra practice?
Resonance is a critical topic in your organic chemistry curriculum. After watching the Resonance Structures Video Series test your knowledge with this short quiz below.
Give it a shot, then check the solutions (linked at the end) and see how you did.
Resonance Structures Practice Question 1
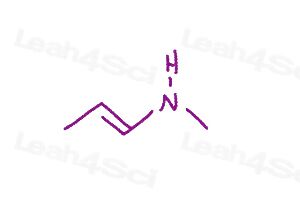
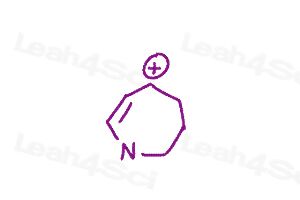
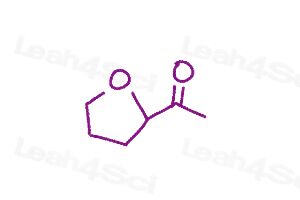
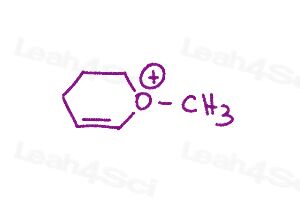
Warm-up question: Draw all major resonance structures for the following polyatomic ions
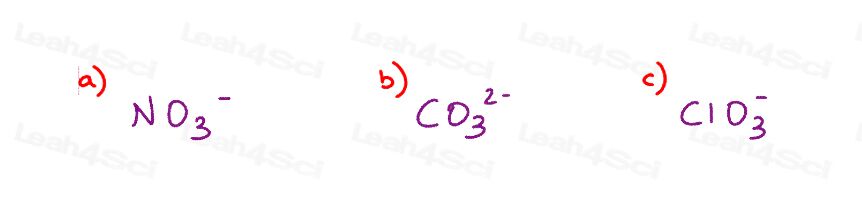
For the step-by-step solution and explanations for Question 1, see my Resonance Practice Problems Video.
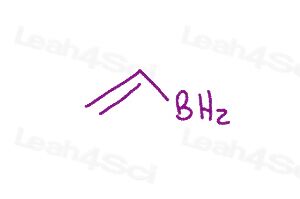
Resonance Structures Practice Question 2
Draw all resonance structures and indicate the major and minor contributors. Pay attention to octets!
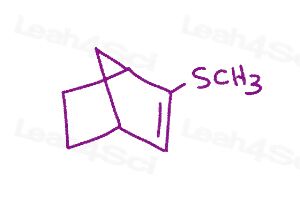
Resonance Structures Practice Question 3
Draw all resonance structures and indicate the major and minor contributors. Don't forget to check for lone pairs.
Resonance Structures Practice Question 4
Show all possible resonance structures, determine major and minor contributors, and WHY.
Resonance Structures Practice Question 5
Show all possible resonance structures. Hint: Pay attention to octets
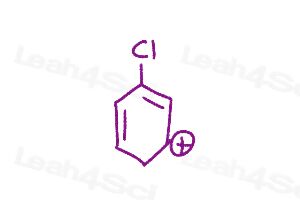
Resonance Structures Practice Question 6
How many resonance structures can you draw for the molecule below? Are they viable (stable)? why or why not?
Resonance Structures Practice Question 7
Show all resonance contributors for the molecule below. Hint: Pay attention to octets!
Resonance Structures Practice Question 8
How many resonance structures can you draw for the molecule below? Show resonance arrows and determine which is the major contributing structure.
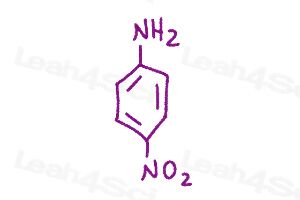
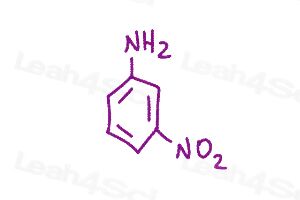
Resonance Structures Practice Question 9 & 10
Using curved arrows show all possible resonance structures for para and meta nitroaniline. Which has more resonance and why?
Need a reminder? Watch the video tutorial on Resonance Structures of Benzene which includes examples of EWG and ERG
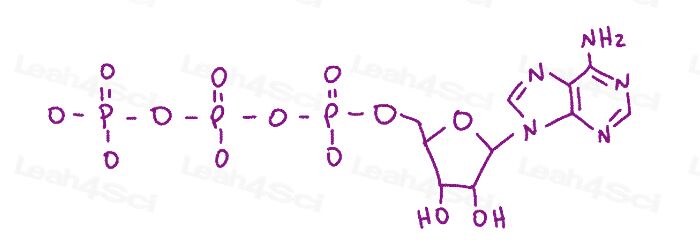
Resonance Structures Practice Question 11
Given the ATP structure below show all possible resonance arrows. Hint: Don't forget formal charges!
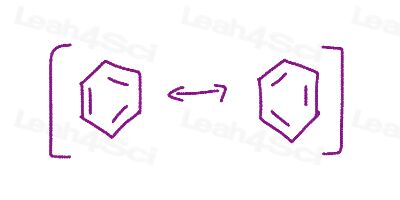
Resonance Structures Practice Question 12
Given the following resonance forms, show how to inter-convert between them using curved arrows, draw the resonance hybrid, and determine which is the major/minor contributing structure.
Need a reminder? Watch the video tutorial on Resonance Structures of Benzene
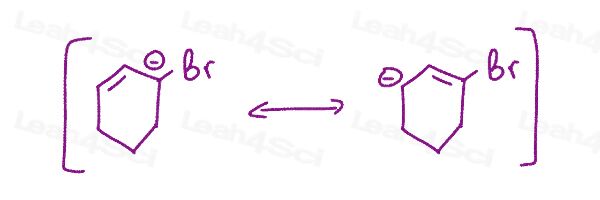
Resonance Structures Practice Question 13
Given the following resonance forms, show how to inter-convert between them using curved arrows, draw the resonance hybrid, and determine which is the major/minor contributing structure.
Resonance Structures Practice Question 14
Using curved arrows, show all possible resonance structures for the molecule shown below
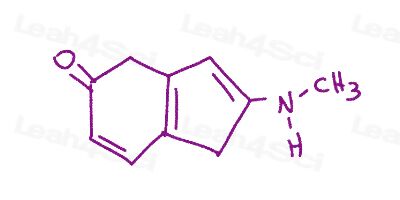
Resonance Structures Practice Question 15
Using curved arrows, show all possible resonance structures for the molecule shown below. Identify the major and minor contributing structures.
Bonus Question:
Show all possible resonance structures using curved arrows and show the resonance hybrid intermediate.