 While many students fear stoichiometry, I hope you’re feeling confident and ready to tackle practice problems after reviewing my MCAT Stoichiometry and Reactions video series.
While many students fear stoichiometry, I hope you’re feeling confident and ready to tackle practice problems after reviewing my MCAT Stoichiometry and Reactions video series.
Haven’t watched it yet? Jump into the video series before attempting the quiz.
This practice quiz was written to test your basic understanding of Stoichiometry and Reactions following along with AAMC Content Category 4E: Atoms, nuclear decay, electronic structure, and atomic chemical behavior.
This quiz is also applicable to students studying stoichiometry in General Chemistry. However, with the MCAT in mind, I chose to skip the nitty/gritty basic questions and instead focus on the types of questions, concepts, and calculations likely to show up on the MCAT.
NO, the MCAT will not ask such straight-forward questions and most MCAT math is no more than 2-3 steps. However, if you are able to complete these problems you can feel assured you've learned how to think and approach related questions (typically just one aspect of a longer question) on difficult MCAT-style passages.
Reminders:
– The MCAT does NOT allow calculators. Not ready for this? Review my MCAT Math Without a Calculator video series.
– ROUND wherever you can, on the MCAT, Close Enough = Good Enough!
– This quiz requires the use of a periodic table (and one IS provided on the MCAT).
Ready to get started? See how you do, then download the PDF solutions (Coming after live quiz review session!).
1- Find the oxidation number of the green atom in each molecule or compound below.
Review: See Oxidation Number video
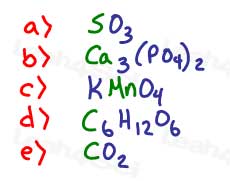
2- Rank the following in order of which contains the LEAST to MOST oxygen atoms:
WITHOUT doing the actual math (don’t find the exact oxygen number). Hint: think in terms of moles.
a) 16 grams of O2
b) 1 mole of sulfuric acid
c) 10 x 10^23 molecules of water
3- Calculate the molecular weight for the following:
Do these ONCE, then commit to memory for the MCAT. Use the Active Writing strategy to memorize.
a) H2O
b) CO2
c) NH3
d) Glucose (C6H12O6)
e) Valine (C5H11NO2)
MCAT Tip: Memorize the average Amino Acid Weight = 110 Daltons (Da). 1 Da = 1g/mol.
4- Find the mass percent of oxygen in the compounds/molecules listed in Question 3
Review: See Percent by Mass Composition video
5- For each redox reaction pictured below, identify the following:
- What gets oxidized
- What gets reduced
- Oxidizing agent
- Reducing agent
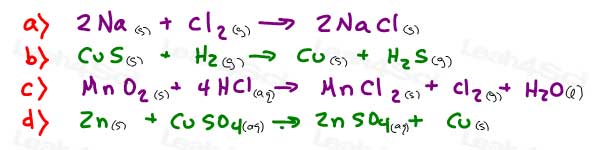
6- Calculate the number of carbon ATOMS in the following:
a) 1g of C-12
b) 1 mole acetic acid (CH3COOH)
c) 3×10^3 molecules of methane (CH4)
7- Find the total mass in grams for each sample in Question 6.
8- How much water is required to create the following solutions?:
a) 0.015 moles glucose in a 0.3M solution
b) 1×10^-4 moles CaCO3 in a 9mM solution
c) 0.3g CaCo3 in a 0.125 M solution
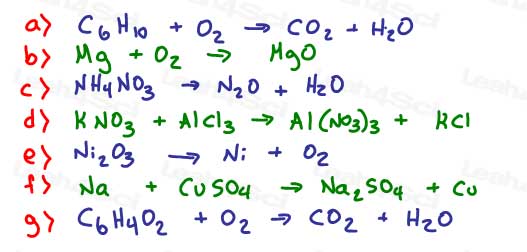
9- Balance each of the following equations and then determine the reaction type.
Review: Reaction types explained in this video, and review these for balancing
- Combination/Synthesis
- Disproportionation
- Single replacement
- Double replacement (metathesis)
- Decomposition
- Combustion
10- Combustion Analysis:
a) How many grams of CO2 are produced from the complete combustion of 100 grams of ethane (C2H6)?
b) How many grams of water vapor form from the complete combustion of 24 grams of glucose?
11- Find the number of moles in the following samples:
a) Oxygen in 25g sulfuric acid
b) Cl in 1.8×10^7 of molecules NaCl
c) CO2 in 0.00109 kg of CO2
12- Find the mass of 30 mL seawater.
(Hint: required knowledge for the MCAT: density water = 1,000kg/m^3 and 1029kg/m^3 seawater)
13- Find the density of 1L of a 0.3molal NaCl solution.
14- Complete and balance the following reactions:
Hint: while formulas are given, you should know these compounds by name for the MCAT. Review balancing acid base reactions
a) Complete combustion of isopropanol (C3H8O)
b) Complete combustion of benzene (C6H6)
c) Neutralization of Carbonic acid (H2CO3) with Potassium hydroxide KOH
d) Neutralization of Acetic Acid (CH3COOH) with Sodium hydroxide
e) Ammonium phosphate (NH4)3PO4 with Calcium Nitrate Ca(NO3)2 (hint: remember your solubility rules)
15- Balance the following redox reactions.
A and B in acidic conditions, C in basic conditions.
Review: balancing redox equations
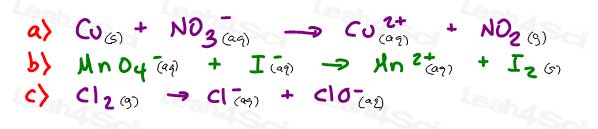
16- Find the molarity and molality of the following solutions:
a) 0.05mg NaCl in 10mL water
b) 47g Iron (II) Chloride in 800mL water
17- Find the Empirical and Molecular formula of Corannulene in the combustion reaction of 6 moles of Corannulene yielding 2.6kg CO2 and 0.27kg H2O.
No, don't google the name 😉 Find the empirical formula first as taught in this video
18- Limiting Reagents and Percent Yield:
a) How much CO2 and H2O will evolve from the combustion of 1.2kg glucose in the presence of 200g O2 gas? Calculate the % yield if just 80g H2O evolves.
b) How much PbBr2 is expected to precipitate when 280g PbCl2 is mixed with 120g KBr? Find the % yield if an experiment yields 50g PbBr2.
How did you do?
Need to Review? Study the Stoichiometry and Reactions Series!
Looking for more? Join the MCAT Study Hall! You’ll have unlimited access to Video Solutions of all the MCAT Practice Quizzes and so much more!
Find an error on the quiz or solutions? Let me know here!!


