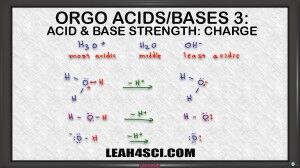
 Charge is the first think to look for when asked to rank acids and bases without a given Ka or pKa.
Charge is the first think to look for when asked to rank acids and bases without a given Ka or pKa.
This video shows you how to compare the charges of the acids, or compare the conjugate bases.
But more importantly, this video shows you how to tackle any acid/base ranking question by comparing conjugates and analyzing for stability.
(click HERE to watch this video on YouTube. Transcript coming soon)
Watch Previous Video: Ranking Acids and Bases with Ka and pKa values
Watch Next Video: Ranking Acids and Bases using Atom Electronegativity or Size
Ready to test your Acid/Base skills? Try my FREE acid/base practice quiz


Thanks so much . I appreciate all of your videos .
Ok these molecules are pretty simple and the explanation was impeccable. My question is how do you identify the acidic hydrogen on more complex molecules?
I just want to say that you are awesome! The way you teach and explain these concepts, which I thought I understood but only truly understood after watching your videos. I literally hear your voice in my head as I go over these concepts again and again 🙂
If the conjugate base has a positive partial charge, is it more stable than a conjugate base with a negative partial charge?
I loved ur concept
Thanks, Dribya!
Finally some clarity. Thanks
Thank you Kristine. Glad I can help 🙂