 Below is the written transcript of my YouTube tutorial video Acids and Bases in Organic Chemistry – Effect of Orbital Hybridization on Acidity and Basicity.
Below is the written transcript of my YouTube tutorial video Acids and Bases in Organic Chemistry – Effect of Orbital Hybridization on Acidity and Basicity.
If you prefer to watch it, see the video HERE, or catch the entire Acids and Bases series HERE
(click here to see the video on YouTube)
[Start Transcript]
Leah here from leah4sci.com and in this video we'll continue our Organic Chemistry acid base discussion by looking at how the Orbital Hybridization plays a rule in Acid Strength.

Say you're faced with a problem that asks you to compare the acidity of these three molecules. We have CH3CH3 or Ethane, CH2CH2 Ethane and CHCH Ethine. First thing you probably want to do is draw it out to make sure that you understand what we're looking at. Here we have the molecules all drawn out and they just look like carbon hydrogen chains with zero, one and two pi bonds. How are these molecules acidic? Any molecule can act as an acid if you take away hydrogen. So it's not necessarily that they're good or strong acids but you can still find a way to compare the acid strength. Now you're actually going to see some reactions in Orgo 1 for Alkynes and then Orgo 2 even more reactions or you're actually looking at the acidic hydrogen sitting on the carbon atom.
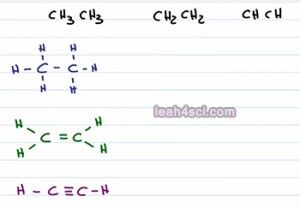
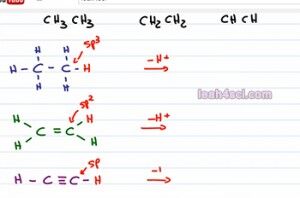
So let's put one red hydrogen on each molecule to show it's acidity, but now what? how do we compare them? The only difference we see here is the number of pi bonds or more specifically the carbon that's holding the acidic hydrogen has different hybridization. For the first one, because we have no pi bonds that is Sp3 hybridized, the second one has one pi bond making it SP2 hybridized and the third has two pi bonds making it Sp hybridized.
If you're not confident with this topic, go back to my hybridization videos on my website, leah4sci.com/orgobasics.
When looking at an Sp3 versus and sp2 and and Sp, these hybridized orbitals are formed by combining the different number of S n P, but you have to remember that the s orbital sits much closer to the positive nucleus. So if I take an acid, pull away hydrogen and give it negative electrons to form a conjugate base the closer I put those negative electrons to the positive nucleus, the more stable it's going to be. So the more s character we have in that hybridization the more acidic the molecule is going to be. S character is simply percent s out of the total number of orbitals. So here's how we calculate it, the sp3 hybrid is made of s, p and p so if we have 1s over 45 total orbitals, that gives us 25 percent s character, that's 1 over 4. The sp2 hybrid is made 1 s and 2p so if we divide 1s by 3 for sp2, we get a total of 33 percent s and finally the sp hybrid is made of an s and a p orbital and that means that if we divide 1s by 2 orbitals we get a total of 50 percent.

This 25, 33 and 50 percent is the s character or the percent s that dominates in that orbital. So if we have only 25 percent s character, that means the conjugate negative electrons are not sitting back close to the nucleus, they're not gonna be as stable. 33 percent s character has more s stabilizing the conjugate a little more, 50 percent s character is about as much as you can get and that means an sp hybrid has those negative conjugate electrons sitting much much closer to nucleus and making it much more stable.
So let's go back to our initial example and show what it looks like after we deprotonate the acidic hydrogen and look at the conjugate bases. Because we remove the hydrogen we have to show the negative and the lone pair of eletrons. For the first one because it was sp3 we have 25 percent s character, the negative is not being stabilized, that means this is going to be a strong base. If you deprotonate an alkane it's going to be so unstable and so so reactive. Definitely keep this in mind when you're discussing nucleophilic attacks or grignards, alright that's a carbonium-like molecule because it's so unstable it's very likely to attack.
For the second one we have the negative electrons sitting on that former sp2 hybridized carbon and that makes it somewhat more stable so we'll call it less strong. And for the last one, because the electrons are sitting on a carbon that was sp hybridized, we have 50 percent s. The electrons are so much more stable and that makes this one weak or we should say the weakest. It's still strong but in comparison to these two molecules it is the weakest and therefore the most stable. When we form a conjugate base that is stable it has to come from a strong acid because the strong acid is most likely to deprotonate to give us that stable conjugate base whereas the strong or unhappy, unstable conjugate base is less likely to deform making that come from a weak acid.
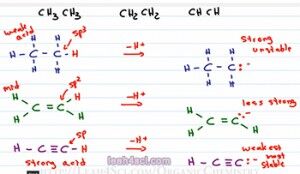
And finally the sp2 is in the middle, it's not as strong as an alkyne but it's not as weak as the alkene. But this doesn't apply just to acids, we can also look at bases in the exact opposite direction. If we're saying that an sp hybridized atom is more acidic than something sp2 which is more acidic than something sp3, we can say that acidity goes to the left from sp3 being the least acidic, sp being the most acidic. Now if acids are inverse to basses meaning the stronger something is as an acid the weaker it is as a base we can say that basicity goes in the exact opposite direction. That means something sp is most acidic but less basic and sp3 which was least acidic is going to be most basic.
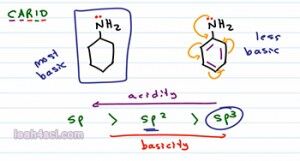
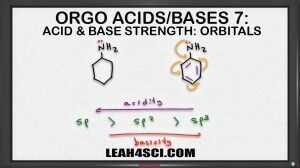
So if I compare these two molecules we have a 6 numbered ring, one with resonance and one without. If we wanna rank them in terms of basicity we can still use CARIO, notice that charge is exactly the same. As a base the nitrogen will use its lone pair of electrons to reach out for proton and in both case is formed positive one so it can rank charge. Atom is the same, the nitrogen is the basic atom for both molecules. Resonance is a factor here eventhough it's not that obvious. The Nitrogen on the left has no resonance but the nitrogen on the right is capable of resonating into the benzene ring. Let's hold off the math, induction also plays a role here because the benzene does have the inductive effect but specifically I want to look at the orbitals. At first glance both Nitrogen atoms appear to be sp3 hybridized but if an atom is capable of resonance, that atom will hybridize to form resonance. So what looks like an sp3 Nitrogen will actually hybridized the sp2 so that it can participate on resonance in the benzene ring. If we keep that in mind, then we can rank them as follows, the Nitrogen on the left is sp3 hybridized making it the most basic because it would be the least acidic and the Nitrogen on the right, because it hybridizes to sp2 is going to be less basic base on hybridization. It'll also be less basic base on resonance because you can think of it as being distracted. So that's two different ways to recognize that the molecule on the left, the one with the sp3 Nitrogen is the stronger base.
Now speaking or Aromaticity, be sure to join me in the next video where we take on more advanced approach to acids and bases by looking at how aromaticity will impact the strength of acids and bases.
Are you struggling with Organic Chemistry? Are you looking for resources and information to guide you through the course and help you succeed? If so, then I have a deal for you, a FREE copy of my eBook “10 Secrets to Acing Organic Chemistry”. Use the link below or visit orgosecrets.com to grab your free copy. After downloading your free copy of my eBook, you’ll begin receiving my exclusive email updates with Cheat Sheets, reaction guides, study tips and so much more. You’ll also be the first to know when I have a new video or live review coming up.
If you enjoyed this video, please click the thumbs up and share it with your Organic Chemistry friends and classmates. I will be uploading many videos over the course of the semester so if you haven’t subscribed to my channel yet, do so right now to be sure that you don’t miss out.
[End Transcript]
Click HERE to Catch my entire video series on Acids and Bases


Leave a Reply