 Below is the written transcript of my YouTube tutorial video Fischer Projections Tutorial Video for Single and Multiple Chiral Centers
Below is the written transcript of my YouTube tutorial video Fischer Projections Tutorial Video for Single and Multiple Chiral Centers
If you prefer to watch it, see Video HERE, or catch the entire Fischer Projections Tutorial Series.
(click here to see the video on YouTube)
[Start of Transcript]
Leah here from leah4sci.com/MCAT and in this video series we'll look at Fischer Projections starting with what they are and how to draw them. And then we'll look at how to convert between Fischer Projections and Sohar structures, Newman projections and even Hayward structures for sugar. You can find my entire series along with a Fischer Projection practice quiz and cheat sheet by visiting my website, leah4sci.com/Fischer.
Fischer Projections were introduced by Dr. Emil Fischer who was studying carbohydrates and used a simpler way to represent these complex chiral 3-dimensional molecules. Imagine that you're working on these reactions and drawing them again and again. If I had to draw the structure again and again, I would lose my mind. But if you look at something like this, it's simpler, it got clean lines and it makes it much easier to study and much less confusing. But Fischer Projections don't apply only to sugars, you can use them to represent all sorts of chiral molecules. We'll start with something as simple as a molecule with one chiral center.
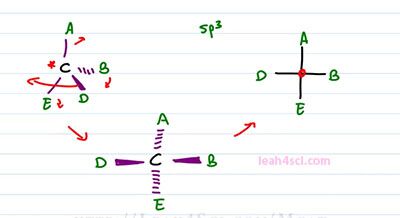
Recall that in order for the molecule to be chiral, it has to have an isometric center which is typically drawing to be a carbon atom. The hybridization is sp3, which means it's tetrahedral and 3-dimensional and it has to have ordinate substituents. For this example, we'll just use letters A, B, D, and E where the center carbon is the chiral carbon. To represent this molecule as a Fischer projection, all we have to do is draw a cross where the lines going up and down represents groups going into the page and the lines to right and left, represent groups coming out of the page. What we show you how to convert it and then I'll show you how to read it.
Converting is a little tricky when you're given a standard structure so you want to re-arrange your molecule so that your 3-dimensional structure shows the group on the right and left coming out of the page and the groups up and down going into the page. In order to do that, we have to get creative and we're going to take the molecule starting at A and spin it. Almost imagine that this is a close book where D represents the top of the book and B represents the bottom and you're grabbing the top page to cover D and opening it in this direction. By doing so, imagine that D comes from the right, up towards you and then towards the left but it's still facing up and out of the page. In the process, we're also moving it slightly upward so it's still out of the page but now it's horizontal instead of pointing down. In the process of pulling D, B got pulled forward and that means instead of pointing into the page, it now points out of the page. And remember, since we tip the molecule slightly upward so that D went from somewhere below the carbon to the level of the carbon, anything on top got pushed back and that means A got dumped into the page. So we show that with dashes for A going into the page. And finally E also got pushed slightly down and that means E is also going into the page. And this is the structure that you want to imagine when you see a simple cross with the substituents around it. As you can see all we did was take the structure, flatten it out so we got rid of dashes and wedges but hidden in that Fischer Projection is still the idea of wedges, horizontal and dashes on vertical.
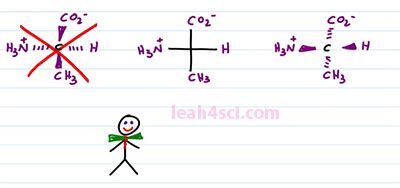
So looking at the Fischer Projection, again here's what you want to recognize, the central carbon, the carbon in the middle of your cross, that is your chiral carbon, that's where the substituents are attached. Anything on the horizontal is coming out of the page to the right and left. Anything on the vertical is going into the page. A goes into the page on top of the molecule and E goes into the page on the bottom of the molecule. When students look at the Fischer Projection, they'll often confuse, is it horizontal wedges or horizontal dashes? Let's explain it using the Amino Acid L-alanine as an example. We have a carboxylate on top an NH3+ on the left, Hydrogen on the right and then the Alanine r group which is the CH3 on the bottom. A lot of students see this and then they get confused. Is it horizontal wedges and vertical dashes or the other way around? The correct way to represent this molecule is a central carbon with a hydrogen atom forward on the right, NH3+ forward on the left, and CO2 minus and CH3 going into the page, top and bottom. What you don't want to draw is something like this, where your carboxylate and your methyl come out of the page on the vertical and the hydrogen and the NH3+ go into the page on a horizontal. And the good trick to remember this is to picture this guy. This here is Mr. Organic Chemistry and he's so excited to join our video that he came just in bow tie and notice that with the bow tie it's horizontal wedges, that means the horizontal is coming out of the page and you can imagine that the vertebrae and his spine that goes up and down and that goes into the page. That is how you want to remember the structure of the Amino Acid. And yes it is very important because not only is this structure incorrect, it's actually the enantiomer of the molecule we started with. L-alanine is D-Alanine which is completely a different structure. Watch what happens if we put them side by side.
The chiral carbon hasn't moved and the substituents are oriented the same way around each one. But I were to superimpose them, they would be exact mirror images, because for every substituent going into the page for L-alanine, it comes out of the page for D-alanine and if it comes out of the page for L-alanine, it goes into the page for D-alanine. When you have two molecules that are chiral, have the same exact constituents but opposite orientation, they are an enantiomers. And this is critical to a biological system where proteins and enzymes are very specific to the structure they recognize. If they're looking for an L-alanine, they will not recognize a D-alanine just like a right hand glove will not fit your left hand because they are not super impossible. And let's prove this by finding R and S for each molecule.We'll start with D-alanine where our substituents are prioritized, Nitrogen, number 1, Carbon with an Oxygen is number 2, Carbon with Hydrogen is number 3, and Hydrogen is number 4. We get rid of our lowest priority substituent, number 4 and then trace the path from 1, 2, to 3.
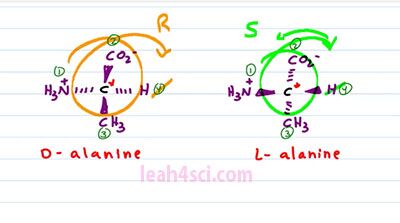
Since the top of the path goes to the right, this is R and then look at the same thing for L-alanine. Nitrogen is number 1, Carboxylic number 2, Methyl 3 and Hydrogen 4. When we cross out number 4, and trace the path from 1 to 2, to 3, it looks like it's also R, but in order to have an R, number 4 has to be in the back. If number 4 is coming out of the page, we have to reverse the chirality because we have to imagine we're looking at it from the other side and that means L-alanine is S where D-alanine is R, proving ones again that these two are enantiomers.
Here's another concept you want to consider with Fischer Projections. And that is what is happens when you do a ninety degree rotation. Let's do a simple molecule where our substituents consist of an OH, a CH3, a Hydrogen and a Chlorine. What happens if we take this molecule and rotate it ninety degrees clockwise or counter clockwise, you will get the same thing. So that now we have OH to the right, CH3 down, Hydrogen left and Chlorine on top. It looks like they should be the same, but they are actually enantiomers of each other and I want to prove it.
Let's try out the 3-dimensional version of this molecule. So we have CH3 and CL coming out of the page. We have Hydrogen down into the page and OH up into the page. That's our first structure. Now let's see what happens with the second structure. But first we'll change the color to make sure we can tell them apart. We'll start with the central carbon, but now we have the OH coming out to the right, Hydrogen coming out to the left, the CH3 is down into the page and the chlorine is up in the page. If we're trying to figure out the relationship just by looking at it, it's hard to tell, because the substituents are not lined up correctly. So we have the option of finding R and S, or we can rotate this molecule exactly as we see it. Because it's not an official projection, I can rotate it as much as I want because molecules can rotate in space and this is a 3-dimensional molecule. I'm going to rotate it ninety degrees counterclockwise so that the atoms below wind up. Once again we have the central carbon, now we have the OH coming up and out of the page because again, I'm keeping the dashes and wedges, I'm simply turning the molecule to a ninety degrees. I have the Chlorine, left and into the page, Hydrogen down and out of the page, and Methyl, right and into the page.
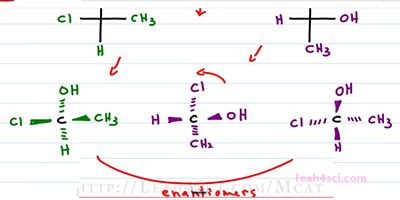
Take a look at these two structures. The oriented, the same way around the chiral carbon but where the purple comes out of the page, the green structure goes into the page, where the purple goes into the page, the green goes out of the page and that means they are enantiomers of each other. So how did that happen? Well, if the Fischer Projection represents dashes and wedges and you rotate at ninety degrees, you're swapping all your dashes and wedges getting the exact mirror image. But if you take that Fischer Projection and flip it upside down, then you have a hundred and eighty degrees meaning it's the same exact thing. Because zero degrees is your starting molecule, ninety is the enantiomer and a hundred and eighty degrees is the enantiomer of the enantiomer or simply your starting molecule.
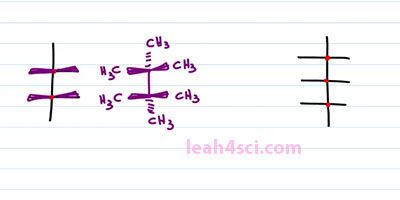
As you're studying Fischer Projections in Organic Chemistry and Biochemistry you'll see more complex molecules that have multiple horizontal lines and your one vertical. We treat it the same exact way and read it the same exact way. For this structure, we have two chiral carbons and the substituents coming out of the chiral carbons will be out of the page for the horizontal and into the page for the vertical. That means we can take the structure and just put wedges on it like this. Any lines on the structure represents bonds between carbons and if it ends in a line, that's a CH3. For example here we have a methyl group, here we have a methyl group or we can choose to put substituents on it. What this will look like is a bond between your two chiral carbons, a methyl group going into the page from the bottom. A methyl group going into the page on top and then methyl groups coming out of the page go from the right and the left. And the same thing applies if you have even more chiral carbons. For example here we have three chiral carbons, if we think on the right and the left is coming out of the page and anything on the top and bottom is going into the page.
For your test questions, we'll likely have you look at molecules that are not in Fischer Projection and have you convert them. Or Fischer Projections ask to compare to something else. So you understand Fischer Projections but now how do you find Chirality if you have one chiral center, two chiral centers or more? That's exactly what we'll be covering in the second video which you can find along with the entire series on Fischer Projections, the Fischer practice quiz and cheat sheet by visiting my website leah4ci.com/Fischer.
[End of Transcript]
Click HERE to Catch my entire video series on Fischer Projections.


