 Below is the transcript of my tutorial video on sp and sp2 Molecular and Electronic Geometry in MCAT Chemistry
Below is the transcript of my tutorial video on sp and sp2 Molecular and Electronic Geometry in MCAT Chemistry
(click here to see the video on YouTube)
[START TRANSCRIPT]
Leah here from leah4sci.com/MCAT and in this series I’m gonna take you through the electronic and molecular geometries when molecule has SP, SP2, SP3 and even SP3d and sp3d2 hybridization. If you’re not comfortable with hybridization go back to my where I show you what a hybrid is and how you get it and you can find this on my website at leah4sci.com/MCATGeometry.
For this demonstration I’ll be using a molecular visions model kit and you can pick your own on amazon by using the link below or visiting leah4sci.com/kit. If you purchase through my link I do get a small commission at no extra cost to you.

We’ll start our discussion with SP hybridization. Notice that when you have S and P we have two groups and that means the atom is going to have two sigma bonds and two pi. When we look at the sp hybridized atom, the sp hybridized atom has two degenerate orbitals made from s and p and since s and p is two fused orbitals we have a total of two groups equidistant from each other since s and p form two degenetized orbitals according to the VSEPR theory, the farthest apart that they can be from each other is a hundred eighty degrees.
Say we have an sp hybridized atom and one of its orbitals is to the right, the farthest that we can place that second orbital is going to be to the left. If this is an atom that’s going to have two bonds, for example Be Cl2, we’ll the Beryllium in the center and on other sides we’ll have a chlorine atom so that we get a linear molecule with a hundred and eighty bond angle.
But if you have an atom that is an sp hybridized with additional empty orbitals on this molecule this is where you start to see your double and your triple bonds. For example the molecule ethine or acyteline is H C triple bound C H. Both carbon atoms are the same so let’s just take a look at one of these carbon atoms which we’ll show in red.
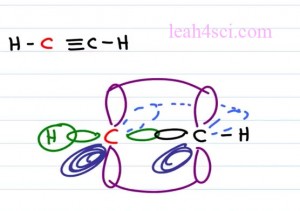
The carbon atom has two degenerate hybrid orbitals, one forming a sigma bond to hydrogen on the left, and the other forming a sigma bond to the carbon on the right. But in addition to the sp hybridization, this carbon atom has two p orbitals. We have one p orbital sitting in the Y plane which is up and down, and that’s going to form a bond with the carbon on the right which also has the p orbital in the Y plane. The second orbital is sitting on the Z plane so to show the Z plane, we’ll have the dark part of the orbital to show that it is coming forward. It’s bold it’s coming right out the page at you and then we’ll show the light one going into the page it’s fading away it’s harder to see and again these electrons will overlap forming that second pi bond.
Now let’s see it in the model kit. Notice we have two carbon atoms bound to each other with two hydrogens on the end. The carbons have a sigma bond in the middle that’s hard to see. We have a carbon in the Y plane and another one in the Z plane, that makes this molecule a hundred and eighty degrees which is linear in both in electronics and molecular geometry.
When we think of sp hybridization, it doesn’t just have to be a molecule that has a triple bond. For example, here we have carbon dioxide with a carbon on the center and two oxygen atoms. Notice that the oxygens each has three groups and that makes them sp2 hybridized but the central carbon is sp with one double bond to the oxygen on one side and the other double bond one the other side. So while the oxygen atoms are sp2 the central carbon is sp with a pibond in the z plane and another one in the Y plane.
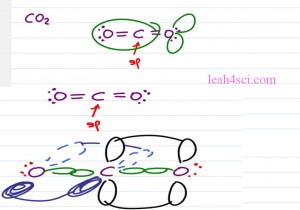
Just like Ethyne, the carbon sp which means is linear and one hundred and eighty degrees. Now let’s see what it looks like on paper.
If we have the molecules CO2 when we draw it out, we have the central carbon atom double bound to two oxygen atoms as far away from each other so once again we have that linear geometry of one hundred and eighty degree bond angle. You can recognize that the carbon atom is sp hybridized because it only has two groups attached to it but the oxygen atoms are each sp2 hybridized because in addition to the molecule attached we have the two lone electron pairs which still take up place which still influence the other electrons around the molecule and so push those away according to the VSEPR theory.
We are focusing on sp for now so let’s take a look at the hybridized carbon and we’ll draw the two oxygen atoms on either side. The carbon is going to have a sigma bond to each of the oxygen atoms but the carbon also has two p orbitals. We’ll show the first one in the Y plane and one of the oxygen atom will be oriented so that its p orbital is also in the Y plane. These electrons can overlap forming a pibond in the Y plane.
The carbon atom has that second p orbital in the Z plane so we show half of the p orbital coming forward and the other half going back. Oxygen atom on the left is going to orient its p orbital in the same direction so that once again we can have a pibond but this time it’s in the Z plane. The oxygen on the right is going to have two electron pairs one forth and back, the oxygen on the left has them up and down. But when you looking at the Molecular Geometry we only look at what we’ll see in terms of the atoms and because the oxygen atoms are as far away from the carbon as possible once again we have the linear geometry and a hundred and eighty degree bond angle.
Now let’s take a look at the sp2 hybridization recognizing that sp2 is made by combining s plus p plus p is sp squared or sp2 giving me three degenerate orbitals which can be occupied as a bond to another atom or as a lone pair of electrons which you can almost imagine as a bond to itself. This will help you see that it takes up space but at the same time there isn’t actually an atom attached over there.
If we wanna see the electronic Geometry for something sp2, just think about that central carbon atom and three groups around it. According to VSEPR theory, the farthest that these three can go is potentially one straight up, one down to the left, one down to the right or any version of this form. Let’s take a look at tertbutyl carbocation as our example. Tert Butyl or Tertiary Butyl is an Isomer of Butane where carbon has three methyl groups bound to it. The tert butyl carbocation gives you a central carbon atom with only three bonds and an empty p orbital, this is how you get the formal charge of plus one.
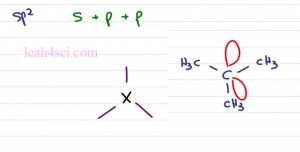
When you look at the actual molecule we’ll just see the carbon and the three groups bound to it. That empty p orbital doesn’t actually take up space and so we don’t account for it in the structure.
So here we have the Tert Butyl cation, notice that we have a carbon in the center, with three methyl groups each center is a carbon and these represent my hydrogen atoms. Notice that the carbon has only three bonds coming out of it with an empty p orbital sitting here but we don’t see it because that’s the nature of the carbocation, fact it’s empty. Notice that at the central carbon, the blue carbon is triangular in shape and flat and that’s why we have a Trigonal Planar Geometry.

If you imagine this as a circle and cut the circle in three, we get a hundred and twenty degree bond angle. Typically when you think of sp2 hybridization, you’re thinking of a pibond. So here we have Acetone or simply prophenol. Here with the oxygen atom where the two red groups are simply the lone pairs of electrons and here we have our two carbon atoms. Notice that the central carbon atoms has three groups coming out of it. Here we have a sigma and pibond and here we have two sigma bonds. Once again this molecule is relatively flat, we still have that triangle shape and that gives us a Trigonal Planar Geometry at a hundred and twenty degrees.

Now let’s take a sideways view of this molecule so you can actually see that pibond. Make that central carbon atom purple and we’ll bind it to a purple oxygen atom. They’re bound first with a sigma bond and the carbon is also bound to a methyl group coming forward so that’s a CH3 involve and then another methyl group going back so that’s a CH3 on a dotted orbital. These are all your sp2 hybri single bonds. With Oxygen we have the same idea but oxygen is only bound to carbon so the other electron pairs you need to imagine as if the oxygen has an electron bound to itself. We have that same orbital coming forward, that same orbital going back but just imagine that there’s an electron bound to an electron and that is how you can invision your lone pair as taking up space and participating in the VSEPR theory pushing the other groups aside.
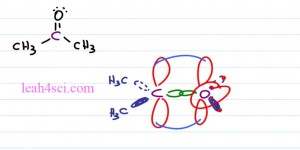
What we still have is that p orbital on carbon and on Oxygen so from a side view we have that flat molecule and it’s pibonds is very high and very low next to the carbon and oxygen atoms. The electronics geometry looking at the electrons and the atoms so both carbon and oxygen would be considered Trigonal Planar but the Molecular Geometry only look at the atoms and for carbon we see Trigonal Planar, for Oxygen all we see is the Oxygen atom.
Be sure to join me in the next video where we go to the sp3 hybridization.
Are you struggling with specific MCAT Topic? I offer Private Online Tutoring where I focus on your needs to strengthen your individual weaknesses. Tutoring details can be found using the link below or by visiting my website leah4sci.com/MCATTutor.
Are you overwhelmed by the sheer volume of information required for the MCAT? Are you worried that lack of a proper study plan and low MCAT score will prevent you from getting into Medical School? My new eBook The MCAT Exam Strategy is 6-Week Guide to Crushing the MCAT will help you formulate a concrete study plan by helping you figure out where you stand now, identify your goals and figure out what it takes to reach them and it’s yours FREE when you sign up for my email newsletter at McatExamStrategy.com. By signing up for my email newsletter, you’ll also be the first to know when I have new videos, MCAT Study Guides, Cheat Sheets, Tips and so much more! The link again McatExamStrategy.com
[END TRANSCRIPT]
Watch The Video Here: sp and sp2 Molecular and Electronic Geometry in MCAT Chemistry


