 How well do you know your Organic Chemistry Basics?
How well do you know your Organic Chemistry Basics?
Start with the Intro to Orgo Video Series. Reviewing these Gen-Chem topics is foundational to Orgo.
Your professor will assume you remember all of these concepts and will plow right on through to the next Orgo topic.
Even though most students forget over Break and fall behind…YOU WON'T! Review and test yourself with the practice quiz below!
It will help you figure out if you’ve mastered this topic.
Download the FREE PDF solutions at the end of the quiz to make sure you are getting it.
Part 1 Atom Basics
Start with the subatomic particles in this tutorial video. Use the linked periodic table to help you answer the questions below.
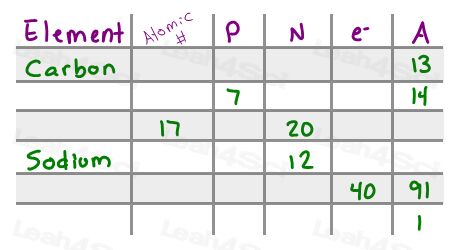
- a) Complete the chart below filling in any missing information for NEUTRAL elements
Element name
Atomic number
Protons
Neutrons
Electrons
Mass number
- b) There are 3 isotopes of Hydrogen. Provide the name, symbol, nuclear charge, and atomic mass for each.
- c) What is the charge of the electron cloud in Chlorine-37?
1. d) What is the nuclear charge of the following elements?
Sulfur
Sodium
Manganese
Xenon
Strontium
Part 2: Periodic table trends.
Review your periodic table trends required in organic chemistry in this tutorial video. This section requires a Periodic Table.
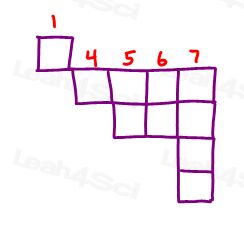
2. a) List all of the elements in group 3A.
2. b) How many elements are in period 6?
2. c) Rank the following elements in order of increasing electronegativity:
Fr Cl P Cs F Al
2. d) Rank the following elements in order of decreasing size:
Br F At Cl I
Can you do this WITHOUT looking at the periodic table?
Hint: You should have memorized this
2 e) Fill in the 10 atoms you are required to MEMORIZE for Organic Chemistry WITHOUT looking at the table. If you have to look do so, then rewrite it 5 times a day, every day, till you have it memorized. YES, MEMORIZED!
Part 3: Atomic Orbitals.
Review your atomic orbitals in this video tutorial.
3. a) Draw the following atomic orbital shapes:
1s
2p
3s
4px
3. b) how many electrons can occupy each of the following orbital or suborbitals
2s
6f
3pz
4th shell (includes all orbitals in the 4th shell)
3. c) draw out the 4d-block of an element with 7 electrons in its 4d shell
3. d) How many p electrons in the following atoms or ions?
S
Be
F-
K+
Fe3+
Part 4: Electron Configuration.
Review electron configuration in this video tutorial and a quick example (in this second video).
4. a) Draw the electron configuration for the following neutral atoms. Your drawing should include the energy levels showing all occupied orbitals and their relative energies.
Carbon
Calcium
Chlorine
Sodium
Cobalt
4. b) Provide the full and shortcut electron configurations for the atoms in the previous question above.
Hint: Shortcut uses kernel for inner shells as taught in this video.
4. c) Which atoms is represented by the following configurations?

4. d) Which cations are represented by the following electron configurations?
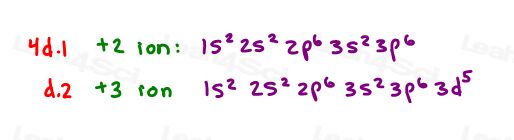
Part 5: Lewis Dot Structure and Octet Rule.
Review the difference between Lewis Dot for atoms (also tested here) and Lewis structure for molecules in the next series.
5. a) Draw the lewis dot structure for the following atoms:
Ca
O
Li
C
Kr
5. b) List 3 elements happy with less than 8 electrons in their ‘octet’ and explain WHY they are an exception to the octet rule.
Bonus: Can you draw molecules showing the octet rule violations?
5. c) List 3 elements happy with MORE than 8 electrons in their ‘octet’ and explain HOW they can disobey the octet rule.
Bonus: Can you draw molecules showing the octet rule violations?
5. d) What IS the octet rule? What’s its purpose as it relates to organic chemistry?
Are you SOLID with your basics?
Do you feel you got the questions correct?
Did you UNDERSTAND how to tackle each question?
Click below to download PDF solutions and see how you scored.