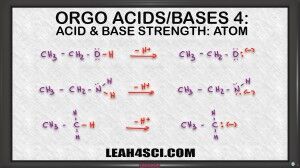
 Video 4 in the acid base tutorial video series shows you how to compare the size or electronegativity of the charged atom. Specifically, you want to look at the atoms on the conjugate base when they gain a charge after losing the acidic hydrogen.
Video 4 in the acid base tutorial video series shows you how to compare the size or electronegativity of the charged atom. Specifically, you want to look at the atoms on the conjugate base when they gain a charge after losing the acidic hydrogen.
It's not just about memorizing when to compare electronegativity, or when to compare atom size, instead the video has a strong focus on helping you understand WHY to compare each aspect in the specific situation. By understanding this you'll be able to apply it to any acid base question on your organic chemistry quiz or exam.
(click HERE to watch this video on YouTube. Transcript coming soon)
Watch Previous Video: Ranking acids and bases C = Charge
Watch Next Video: Ranking acids and bases R = Resonance
Ready to test your Acid/Base skills? Try my FREE acid/base practice quiz



What if one has resonance but the atom’s are not the same — that is, the negative charge is on an oxygen atom on one structure and the other structure has the negative charge on a carbon. The latter, however, is capable of resonance. Does resonance make the carbon second structure more stable?
We can use the acidity and basicity trend also right?
Acidity/basicity trends come FROM size and electronegativity trends.
What if it is neither in the same group nor in same period, then what should we compare ?
You have to make a decision based on what you’re given. Most professors won’t get too evil with this