Alkene reactions are the foundation for all future organic chemistry reactions and mechanisms. To help you build that solid foundation I've put together this short quiz testing your knowledge of reactions, reagents, products and additional molecule concepts.
These questions range from medium to tricky and should be completed AFTER you've studied my Alkene reaction video series since each video already contains a few simple practice problems.
Take your time and think through each question. If you're not sure how to proceed click the ‘hint' link for the related mechanism tutorial video. Once finished scroll down and grab a free copy of the solutions.
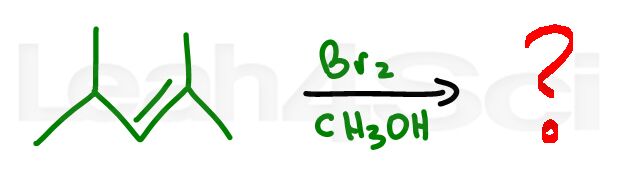
Alkene Practice Question 1
Identify the reagent and solvent required to carry out this reaction

Hint – if you're stuck on this reaction watch this video to learn the mechanism
Alkene Practice Question 2
Identify the product formed when this alkene reactions with Cl2 in water. Pay particular attention to stereochemistry.
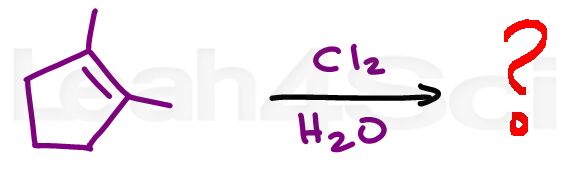
Hint: There may be more than 1 product. For help with this reaction click to watch the mechanism video
Alkene Practice Question 3
Identify the product formed when the following alkene is reacted with BH3 and THF, then followed up with H2O2 and NaOH
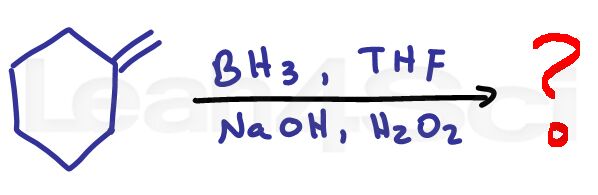
Hint: This is an anti-markovnikov reaction.
Alkene Practice Question 4
Identify the starting alkene that will yield the product shown when reacted with chlorine in CCl4. Pay particular attention to the stereochemistry of product.
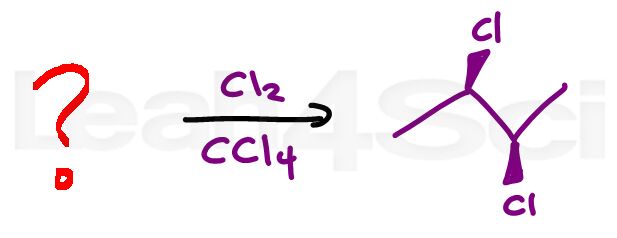
Hint: Stuck on the stereochemistry of this question? Watch the reaction video here to review this reaction
Alkene Practice Question 5
Identify the reagent and solvent required to transform the starting cycloalkene to the given ether.
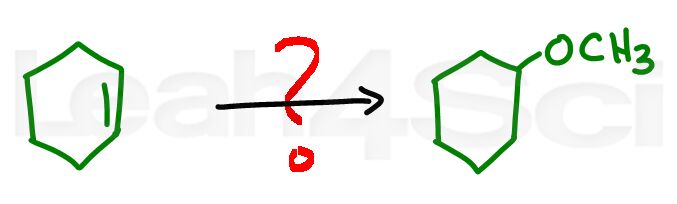
Hint: Water and alcohol undergo similar reactions. Click for the mechanism video
Alkene Practice Question 6
Identify the product when the following alkene reacts with Hg(oAc)2 in alcohol followed by NaBH4
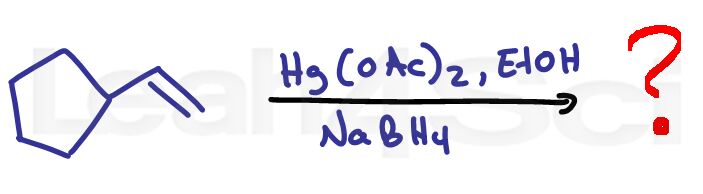
Hint: Pay close attention to each of the given reagents. Stuck on this reaction? Click for the reaction video
Alkene Practice Question 7
Identify the product formed with the given bicycloalkene reacts with Bromine in CH2Cl2. Be sure to include all possible products.
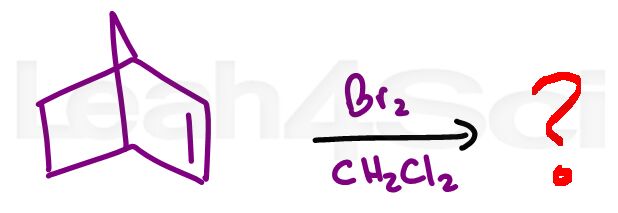
Hint: Don't let the complexity of the molecule throw you off. If you know this reaction you'll get the product
Alkene Practice Question 8
Identify the product formed when this alkene reactions with sulfuric acid in an ethanol solution
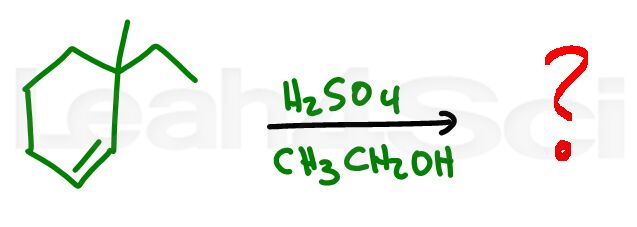
Hint: Pay special attention to the reagents, solvents, and stability of the intermediates. Stuck? review this video
Alkene Practice Question 9
Identify the product formed when the given alkene reacts with hydronium

Hint: Pay special attention to the stability of the reaction intermediate. Need help? watch this video
Alkene Practice Question 10
Identify the reagents required to bring about this alkene to alcohol transformation.

Hint: Consider how the intermediate impacts the regioselectivity of this reaction. Stuck? Watch this video.
Alkene Practice Question 11
Identify the product formed when the alkene below reacts with bromine in a methanol solution.
Hint: Consider how the solvent impacts the intermediate. Need help? Watch this video
Alkene Practice Question 12
Identify the product when the alkene below reacts with sulfuric acid in water.
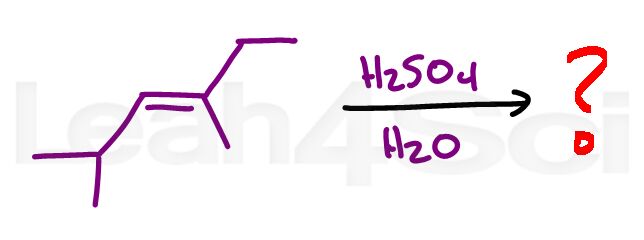
Hint: Don't overthink it. Sometimes tricky looking reactants yield simple products. Tutorial video here.
Alkene Practice Question 13
Identify the product when the following alkene reacts with HCl in CCl4.

Hint: This is a fun (read TRICK) question. Pay careful attention to the logic BEHIND Markovnikov's rule! Click HERE to study the reaction mechanism.
Alkene Practice Question 14
Identify the product when the alkene below reacts with HBr.
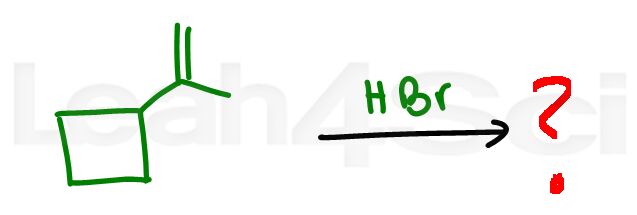
Hint: Other than degree of substitution, what ELSE can cause a carbocation rearrangement? Need help? Watch this video.
Alkene Practice Question 15
Identify the product when the alkene below is reacted with sulfuric acid.
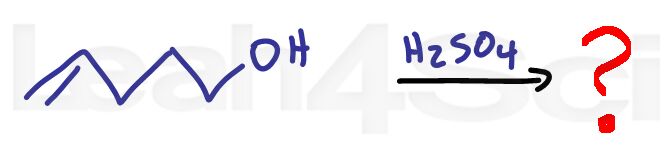
Hint: There is no given solvent, don't make any assumptions 😉
How did you make out?
Compare your solutions to the answer key. Click the button below for details