Amino acids are critical for the MCAT as well as your biology and biochemistry courses. Amino acids are the building blocks of living things, playing key roles in cellular structure, function, and so much more.
This tutorial series will walk you through the different aspects of amino acids as required for the MCAT.
Make sure to test your knowledge afterwards with my MCAT Amino Acids Practice Quiz.

Amino Acid Structures
Despite what your book may claim, you MUST MEMORIZE YOUR AMINO ACIDS! Students who've taken the new MCAT confirm that you must know the following for each amino acid:
- Full Name
- 3 letter abbreviation
- 1 letter code
- Side chain structure and characteristics
- Charge/behavior at different pH values
- and so much more…
Already know the basics?
Watch this video to memorize ALL 20 Amino Acids in just 10 minutes:
Not ready for 👆? Let's start with the basics.
 Video 1 – Introduction to Amino Acids
Video 1 – Introduction to Amino Acids
Before you dive in to memorize side chain characteristics, ask yourself WHY?
What is so special about this biomolecule? What is the logic behind ‘alpha carbonyl' and why are there polar and non-polar, acidic and basic side chains. This video will help you set the stage for the entire series.
 Tutorial: Understanding Amino Acid Side-Chain Characteristics
Tutorial: Understanding Amino Acid Side-Chain Characteristics
It's not enough to memorize names and structures.
You need to UNDERSTAND how each specific R-group contributes to that groups chemistry and participation in 3-dimension structures and reactions.
This tutorial breaks down the side chain groups to help you understand their individual classifications.
 Hydrophobic Non-Polar Neutral Amino Acids
Hydrophobic Non-Polar Neutral Amino Acids
These amino acids may appear out of place in an aqueous biological environment, but they are in fact critical to completing the amino acid picture.
Hydrophobic amino acids feature water fearing neutral and non-polar side chains. This video explains their names, and side chain functional groups/structure.
 Polar, Acidic and Basic Amino Acids
Polar, Acidic and Basic Amino Acids
Hydrophilic amino acids are rather ‘exciting' and reactive compared to the hydrophobic groups.
This video walks you through the polar neutral, acidic, and basic amino acids and their side chain characteristics.
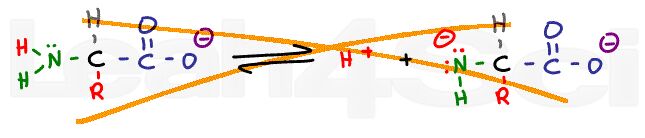
Zwitterions:
Ever notice that amino acids are written with both a positive N and negative O in the same structure? Why? How do you determine the charge of any group within an amino acid or polypeptide?
How does all this relate to the Isoelectric Point or pI?
Tutorial: Amino Acid Charge – Zwitterions and Isoelectric Point
Amino Acid Stereochemistry R/S, D/L, Fischer Projections
Amino Acids are biologically active molecules. 19 of the 20 common amino acids have a chiral alpha carbon. This video shows you how to find R and S configurations on 3D molecules, as well as how to find R/S and D/L for Fischer projections. We’ll compare R/S to D/L and learn how to quickly convert between Fischer and 3D structures.
With plenty of examples, you'll learn to do it the right (read: long) way. But, you'll also learn my quick amino acid chirality shortcut.
 Zwitterion and Amino Acid Charge
Zwitterion and Amino Acid Charge
Zwitterion or ‘double ion' refers to a molecule with a net neutral charge despite wielding a positive and negative charge.
This video shows you how to recognize the zwitterion, how to find it's ideal pH, and how to find amino acid charge at ANY pH.
Peptide Isoelectric Point with Shortcut
The isoelectric point, or pI, of a peptide is the pH at which the zwitterion exists. In other words, it's the pH at which the peptide has a net neutral charge.
This video shows you how to compare the pH and pKa values to determine if an ionizable group is protonated or deprotonated. It also includes my shortcut for quickly finding the pI on a peptide chain with 2 or more ionizable side chains.
Strecker Synthesis of Alpha Amino Acids
Strecker Synthesis is the earliest known method to synthesis an alpha amino acid.
This video walks you through the overall reaction followed by a step by step breakdown of key mechanism components. This is one of the 2 amino acid synthesis reactions required for the MCAT.
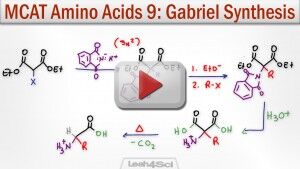 Gabriel Malonic Ester Synthesis of Amino Acids
Gabriel Malonic Ester Synthesis of Amino Acids
This reaction is another popular method for synthesizing alpha amino acids. This reaction is a combination of the Gabriel Amine Synthesis and the Malonic Ester Synthesis reactions.
This video breaks down the overall reaction followed by a walk-through of key mechanism components.
This is the second of 2 amino acid synthesis reactions required for the MCAT.
Peptide Linkage and Hydrolysis Reactions of Amino Acids
Amino acids are the building blocks of living things. This makes the process of making/breaking bonds critical to understanding biological sciences.
This video walks you through the formation of an amino acid bond – the peptide bond, as well as breaking the bonds through hydrolysis.
Even More Tutorials To Follow:
- Disulfide Bridges
Ready to test your knowledge of amino acids? Try the Amino Acids Practice Quiz
Don't forget to download the FREE Amino Acids Cheat Sheet Study Guide