EAS Series: Video 6 This video shows you the mechanism for the formation of carbocation in a Lewis Acid as the super electrophile that is attacked by benzene in the Friedel-Crafts Alkylation reaction. This reaction produces an alkylated benzene. (Watch on YouTube: Alkylation. Click CC on bottom right for transcript.) <– Watch Previous Video: EAS […]
Organic Chemistry Tutorial Videos
Aromatic Sulfonation Reaction and Mechanism Tutorial Video
EAS Series: Video 5 Aromatic Sulfonation is an Electrophilic Aromatic Substitution (EAS) reaction which adds an SO3H to the benzene ring. This reaction can be carried out starting with SO3, or creating SO3 using a heated H2SO4 solution. The video below shows the complete mechanism from creating a super electrophile (SO3) to sigma complex and reforming the benzene […]
EAS Aromatic Nitration Reaction and Mechanism Video
EAS Series: Video 4 This video shows you the mechanism for the formation of Nitronium – the super electrophile that is attacked by benzene in the nitration reaction. This video also helps you understand the role of the acid catalyst before and during the reaction. (Watch on YouTube: Nitration. Click CC on bottom right […]
EAS Aromatic Halogenation Reaction and Mechanism Tutorial Video
EAS Series: Video 3 This video will start by showing you a quick comparison of alkene halogenation versus aromatic halogenation: bromination and chlorination. This video will show you the aromatic halogenation mechanism from the role of the Lewis Acid catalyst and formation of the super-electrophile, through the entire mechanism of adding halogen to benzene. Also […]
EAS Mechanism and Sigma Complex Resonance Video
EAS Series: Video 2 The Electrophilic Aromatic Substitution reaction starts when one of the pi electrons on the benzene rings breaks as it reaches for the super electrophile. While this is similar to alkene reactions AT FIRST GLANCE the remainder of this mechanism is very different and unique. The sigma complex intermediate is capable of […]
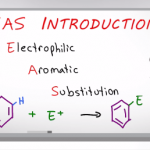
Introduction To Electrophilic Aromatic Substitution
EAS Series: Video 1 The topic of EAS or Electrophilic Aromatic Substitution reactions is one that covers a key reaction pathways studied in the average organic chemistry course. While the mechanism undergoes a broken pi bond and addition to the former sp2 carbon atom, the EAS reaction is very very different from the Alkene Addition […]
Choosing Between SN1 and SN2 Reactions Part 1
SN1 SN2 E1 Series: Video 15 Knowing that your alkyl halide will undergo nucleophilic substitution is not enough. As you work through these reactions pay attention to the key factors that help you distinguish between unimolecular and bimolecular substitution reactions. This is SN1 vs SN2 video one. Additional examples in the next video (Watch on […]
SN2 Reaction vid Bimolecular Nucleophilic Substitution Part 3
SN1 SN2 E1 Series: Video 14 Bimolecular substitution is a fast reaction which requires a good leaving group. Or at least one that can be kicked out easily. However, when faced with a bad leaving group, you must first ‘bribe’ the atom turning into a more willing leaving group before proceeding with the reaction. This […]
SN2 Reaction Chirality and Mechanism of Bimolecular Substitution Part 2
SN1 SN2 E1 Series: Video 13 When starting with a chiral alkyl halide, the SN2 reaction will undergo a backside attack and thus an inversion in chirality. This video shows you a breakdown of the chiral inversion to help you understand how easily to identify chiral SN2 reaction products. (Watch on YouTube: SN2 Part 2. […]
SN1 Reaction Mechanism with Hydride Shift and Carbocation Rearrangement Part 3
SN1 SN2 E1 Series: Video 11 In this final SN1 video you’ll see tricky examples involving less substituted carbocation intermediates followed by carbocation rearrangements and hydride shifts. When working through these reactions pay special attention to the patterns that help determine the SN1 mechanism over a potential SN2 or E2 (Watch on YouTube: SN1 Part […]
- « Previous Page
- 1
- …
- 9
- 10
- 11
- 12
- 13
- …
- 15
- Next Page »