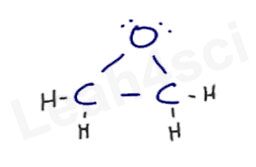
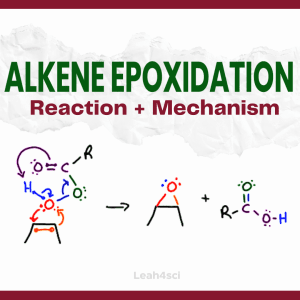
 Reaction Overview: Alkene epoxidation is a reaction in which the pi bond of an alkene breaks and both of the former sp2 carbon atoms bind to the same oxygen atom, forming a 3-membered ring known as an epoxide. This reaction requires a peroxycarboxylic acid, such as m-CPBA.
Reaction Overview: Alkene epoxidation is a reaction in which the pi bond of an alkene breaks and both of the former sp2 carbon atoms bind to the same oxygen atom, forming a 3-membered ring known as an epoxide. This reaction requires a peroxycarboxylic acid, such as m-CPBA.
Epoxidation happens via a concerted reaction mechanism and is considered a syn addition with the oxygen ‘grabbing’ both carbon atoms on the same side of the pi bond.
Epoxides can also be formed from alkenes using a halohydrin intermediate.
Summary of Alkene Epoxidation
- The alkene’s pi bond attacks the ‘extra’ oxygen on the peroxy acid.
- Oxygen uses its lone pair to retaliate in a concerted reaction mechanism.
- The peroxy acid that participates in the cyclic flow of electrons forms a carboxylic acid.
- The final products are an epoxide along with a carboxylic acid by-product.
A note on m-CPBA:
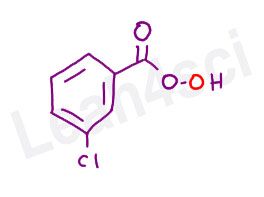
m-CPBA stands for meta-chloroperoxybenzoic acid, or 3-chloroperbenzoic acid.
While it may sound scary and look confusing, this molecule is simply a peroxycarboxylic acid, with a ‘bigger group’ in the form of chlorobenzene attached.
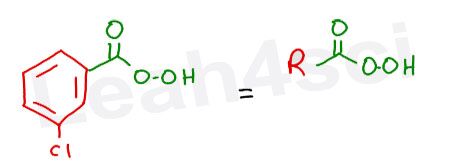
What we care most about in this reaction is the ‘peroxy’ portion. It’s the extra oxygen on the carboxylic acid which makes it reactive for an epoxidation.
Key Reaction Notes
- The ‘per’ in peroxy or peracid refers to an extra oxygen atom with a lower oxidation number. While oxidation numbers are not discussed in orgo, it does help explain its instability and resulting reactivity.
- The product of this reaction is an epoxide, a ring composed of 2 carbon atoms and 1 oxygen atom.
- This reaction prefers the less hindered face of the pi bond.
Epoxidation Mechanism Overview and Step-By-Step Explanation
Professors will often attempt to draw the mechanism with the alkene and peroxyacid next to each other as shown here.
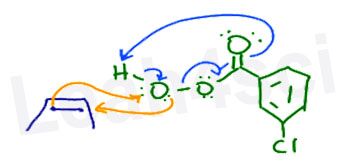
I prefer to line them up next to the specific atoms they will attack as I show further down. This allows for a clean flow of electrons on paper and less confusion overall.
Preparing to React the Alkene with m-CPBA
We’ll use the common m-CPBA for this reaction. Before we attempt the mechanism, let’s line up the molecules so that the extra oxygen (near the H) is ready to be grabbed by the alkene.
I like to think of this reaction as the alkene carbons both reaching up to skewer the oxygen.
That's how I envision the reaction if I’m not asked for a mechanism.
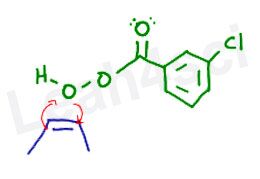
Peroxy Reactivity
To really understand this reaction, it’s important to recognize the extreme reactivity of the peroxy bond. When oxygen is single bound to another oxygen atom, they have a lower oxidation state, which makes them both very unhappy and very unstable. This instability makes them susceptible to reaction. Think of the initiation step in radical reactions when using H2O2 or ROOR in the presence of heat or light.
Concerted Reaction
This is a concerted reaction mechanism. Unlike previous mechanisms where we have a clear ‘step 1’ followed by ‘step 2’, all of the electrons in this reaction flow at the same time in a circular manner.
Imagine that you are directing a game where you have 16 kids sitting in chairs that form a circle.
And you tell the kids:
“On my mark, everyone stand up and prepare to move.”
And they all stand up when asked. Then:
“On the count of 3, everyone take a step to the right.”
And they all do when you count to 3.
Every child is now standing in front of another chair.
Who moved first? Who caused the other kid to move?
If they moved on the count of 3, then EVERYONE moved at the exact same time in a circular fashion, and no one kid moved faster than the other.
(And hopefully no fighting/shoving broke out in the process.)
If the electrons are the kids in the above example, here’s how it happens.
In a concerted mechanism, all of the electrons flow in a circular pattern at the same time.
I numbered my arrows and steps below for the simple purpose of allowing you to connect the words to the image.
Also, pay attention to the color of each atom or electron pair so that you can trace its fate from reactant to product. If unclear, make sure to watch the alkene epoxidation video.
The Alkene Epoxidation Concerted Mechanism
- One of the pi-bound carbons uses the pi electrons to grab the terminal oxygen on the peroxy acid.
- Oxygen uses its lone electron pair to grab the other pi-bound carbon.
This binds the oxygen to both carbon atoms as the pi bond is broken.
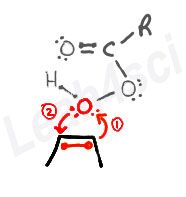
The Peroxy Acid to Carboxylic Acid Concerted Mechanism
At the same time, the extra oxygen has to break away from the peroxy acid. Once again, this happens as a concerted mechanism.
Here’s how I like to think of it:
When the extra oxygen is attacked, the rest of its former ‘family’ wants nothing to do with it. Here are my arrows below, numbered to make them easy to follow AND NOT due to the sequence of events.
- The unstable bond between the 2 oxygen atoms breaks away and moves towards the carbonyl carbon. This forms a new pi bond between oxygen and carbon.
- This action puts too many bonds on carbon, forcing it to release the pi bond between itself and the double-bound oxygen atom. Instead of collapsing onto oxygen, these electrons reach out to grab the terminal hydrogen atom (sitting on the peroxy oxygen), forming a new bond between oxygen and hydrogen.
- Hydrogen can only have 1 bond to fill its 1s shell. When hydrogen is grabbed by oxygen, it must let go of its original bond. Hydrogen releases those electrons back to the oxygen atom.
And that’s it!
Now that you have 2 cycles of flowing electrons, redraw your atoms to show your final products. I prefer to keep my atoms in the same location and simply show all the new bonds that form as a result of the flowing electrons.
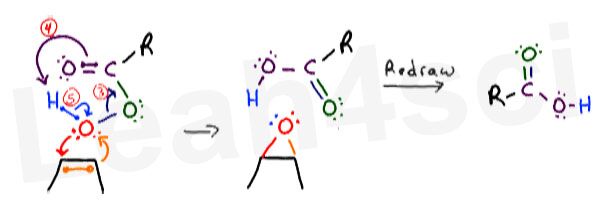
The carboxylic acid is not immediately apparent, but realize that you now have a carbon atom double-bound to 1 oxygen and single-bound to another. You can redraw the products once more to simplify the molecules for a nice and clean final product.
Working on additional reactions? See my Alkene Reactions + Mechanisms Series, and be sure to download the Alkene Reactions Cheat Sheet.
Ready to test your knowledge?
Click to try my free Alkene Reactions Practice Quiz.
Need to see it all in action?