Reaction Overview: The Halohydrin formation reaction involves breaking a pi bond and creating a halohydrin in its place. Halo = halogen and Hydrin = OH. This reaction takes place in water and yields an anti-addition reaction which follows Markovnikov's rule.
Reaction Summary
- Nucleophilic pi bond attacks halogen molecule, pi bond breaks in the process
- Halogen lone pair retaliates – attacks carbon forming a halogen bridge
- Second halogen breaks away as negative halide – immediately surrounded by solvent molecules
- While halide ‘caged' by water, another water molecule acts as nucleopile and breaks halide bridge
- If asymmetric, water attacks at the more substituted carbon
- Yet another water molecule removes the excess H-atom from the attacking H2O
- Product is a halohydrin with halogen on one carbon and OH on its neighbor
Mechanism Overview And Explanation
Asymmetric halohydrins have the OH on the more substituted carbon and the halogen on the less substituted carbon. This follows Markovnikov's rule due to water opening the halogen bridge at the more partially positive carbon atom.
(Watch my Halohydrin Formation Video to see the reaction details come to life)
Key Reaction Notes:
- Halohydrin comes from Halo = Halogen and Hydrin = OH
- Despite lack of carbocation intermediate, this reaction follows Markovnikov's rule
- The more substituted bridged carbon holds more partial positive charge
- Water attacks from the ‘anti' or opposite side of the halogen bridge
- If carried out in an inert solvent halogenation occurs forming a vicinal dihalide
What Exactly Is Happening In This Reaction?
The halohydrin formation reaction is a viable starting point for many multi-step synthesis reactions. Placing a reactive group along with a leaving group on the same molecule allows for chain elongation, oxidation, epoxidation and more.
Understanding The Reactants:
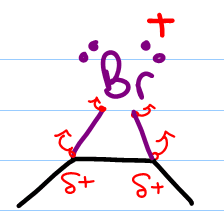
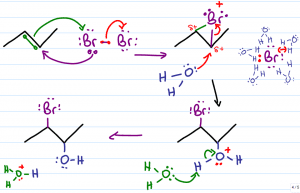
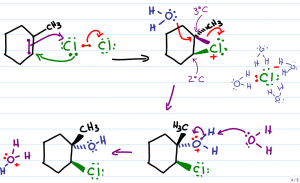
We'll use Br2 for our explanation but realize that this can also take place with Cl2. At first glance, the product of this reaction is not very obvious. In fact, this reaction starts out just like the alkene halogenation mechanism. The nucleophilic pi electrons attack a neutral bromine molecule. The attacked bromine atom retaliates and attacks the carbon with one of its lone electron pairs. The second Br breaks away as a negative bromide in solution.
The two former sp2 (pi bound) carbon atoms are now each attached to bromine with a sigma bond forming a 3-atom ring. The bromonium bridge has an unstable positive charge. To compensate, the bromonium ion pulls on the bonds that connect it to carbon in the hopes of balancing its positive charge with the extra electron density. This leaves the bromine slightly ‘less' positive but also places a partial positive charge on the 2 bound carbon atoms.
The Caged Bromide Ion:
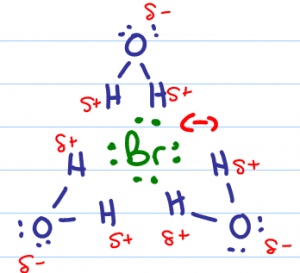 The negative bromide in solution would like to attack the partially positive carbon atom, but it can't. Given that this reaction does NOT take place in an inert solvent, we must account for the water's interference.
The negative bromide in solution would like to attack the partially positive carbon atom, but it can't. Given that this reaction does NOT take place in an inert solvent, we must account for the water's interference.
Water is a highly polar molecule with a partially negative oxygen atom and 2 partially positive hydrogen atoms. The partially positive hydrogen atoms surround and ‘cage' the bromide rendering it incapable of attack.
With the bromide ion out of the way, the next best nucleophile in solution swoops in to attack the partially positive carbon atoms. One of the water molecules will use its nucleophilic lone electron pairs to attack the partially positive carbon.
Formation of the Halohydrin
Water must approach from the opposite face of the molecule given that the bromonium bridge will stand in the way of attack from the same face of the molecule. This is the anti-addition.
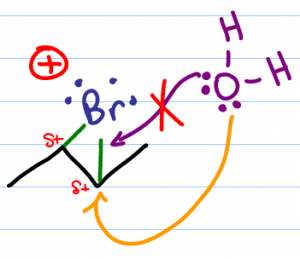 When water attacks carbon, carbon lets go of the electrons binding it to bromine. (bromine wanted them anyway) thus breaking the bromonium bridge.
When water attacks carbon, carbon lets go of the electrons binding it to bromine. (bromine wanted them anyway) thus breaking the bromonium bridge.
The attacking oxygen atom still has 2 bound hydrogen atoms for a total of 3 bonds and one lone pair. This results in an oxonium, or an oxygen atom with a positive formal charge.
Oxygen, an electronegative atom, is not thrilled with the positive charge and therefore pulls on the electrons binding it to hydrogen. This results in a slightly less positive oxygen, and a partially positive hydrogen atom.
Another water molecule in solution reaches out with one of its nucleophilic lone pairs to grab the partially positive hydrogen atom. The electrons break away and return to the oxygen giving it a second lone pair and neutral charge.
This resulting product is a halohydrin. If bromine you get a bromohydrin, if chlorine you'll get a chlorohydrin.
How Markovnikov's Rule Factors In:
Recognize that at no point in this explanation did I mention a carbocation. So why do we still follow Markovnikov's rule? The answer is in the partial charges. After all, Markovnikov's rule is all about creating the most stable carbocation intermediate. When the unstable bridged halogen pulls on its bonds to carbon, the pull will not be equal. If one of the bridged carbons is more substituted, it is more capable of holding a positive charge if a carbocation to form'. And so, even if there is no obvious carbocation forming, the fact that the more substituted carbon is capable of holding the charge, means that it will hold a greater amount of the partial positive charge.
Picture this scenario. You require an important task to be carried out and you have two willing participants. You know that one of your volunteers is more capable of completing the task. I assume you will assign this person the task in place of the other. Same goes for the carbocation.
Now when the nucleophilic water molecule comes to attack, it feels a stronger attraction towards the more substituted carbon. This of course is a direct result of its greater positive charge, and thus still follows Markovnikov's rule
See the Halohydrin Formation Reaction come to life along with practice examples in my tutorial video below:

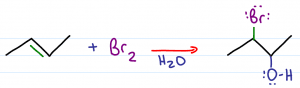


thx for easy breakdown of each reaction. really helps when a lot of orgo professors have no structure to their class or “how to study for orgo”. they just say to do a lot of problems.
You are the Goddess of Organic Chemistry. I No teacher explains it this simple.
Thank you Leah!
The description is really helpful.No one can explain it in a short and sweet manner as you have did it.Can I know which carbon does the hydroxyl group attack after the formation of!three membered ring.Thanks a lot in advance.
why halohydrins is not a type of hydration reaction like oxymecuration and hydroboration..since its reacting in the presence of h2o
Couldnt understand teacher but you r the best! I love your phrase
could you plz explain the mechanism of Isoflurane synthesis from Tri-Fluro-Ethanol? Isoflurane is an inhalation anesthetic.
How do you know whether the OH or the Br ends up on the most substituted carbon?
Can you explain addition of HOX (X-halogen) on alkene
You are awesome. Exceptional explanation. I found myself in the world of water and bromine will reading.
Amazing explanation…your work is really worth appreciating.
what exactly do you mean by a halide bridge?
So there aren’t any halide or alkyl shifts in this reaction mechanism?
I follow all of your lesson and its very helpful. Anw, some books says H from H3O reacts to Br-. And form HBr, it is possible?
wow you are so awesome! no one actually takes the time to go into detail and explain it in a simple way. Really, Thanks!
Thank you Giselle, glad to help
Thank you so much….I got an answer to my query… Your explanation is amazing Thanks once again
Why and how the formation of 3 membered ring takes place?
Lakshita: watch the halogenation video first as it’s explained there
Hi Leah,
Really great lesson, loved the description of what was happening. But could you possibly explain why the CH3 goes from having the dashed away bond, to the coming out thick bond when the H20 joins?
Thanks in advance and great work! 🙂
The backside attack pushes on of the groups to the opposite direction. Dashes = back, gets pushed forward on a wedge
Amazing detail and answered every single question I had about this mechanism. I was especially curious as to how this followed Markovnikov when it looked more like Anti-Mark and you hit the nail on the head with your description. Thank you so so so much 😀
Thank you so much for the feedback and I’m glad I was able to help you understand the reaction. Are you currently in Orgo 1?
thank you so much!! Us O-chem students really appreciate it 🙂
You are very welcome
Thank you again for all this work done on the cheat sheets! WOW! Organized! What a new way to do Ochem…… 🙂 (my desk is a horror scene)
Thank you Angelina,
I’m glad you’re finding it helpful. Do you have an exam coming up? If so which topics?
i realy enjoy the lesson i like you
Thank you Raj