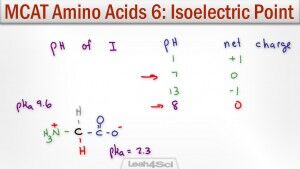
 Isoelectric point calculations are critical in your biochemistry course and MCAT prep. The isoelectric point or pI gives you the pH at which the molecule has a net zero charge. This comes from a balance of protonation and deprotonation so that all positive and negative charges cancel out.
Isoelectric point calculations are critical in your biochemistry course and MCAT prep. The isoelectric point or pI gives you the pH at which the molecule has a net zero charge. This comes from a balance of protonation and deprotonation so that all positive and negative charges cancel out.
This video shows you how to calculate amino acid isoelectric point along with a shortcut to help you figure out WHICH pKa to use when given a side chain pKa value.
(Watch on YouTube: Isoelectric Point of Amino Acids. Click cc on the bottom right for video transcript)
< — Watch Previous Video: Zwitterion and Amino Acid Charge at Given pH value
— > Watch Next Video: Peptide Charge and Isoelectric Point Shortcut
This is video 6 in the Amino Acids Series. Click for complete series + Practice Quiz and Cheat Sheet
Have you grabbed the FREE Printable Cheat Sheet to follow along? CLICK HERE


