 Naming Organic Compounds Series: Video 7
Naming Organic Compounds Series: Video 7
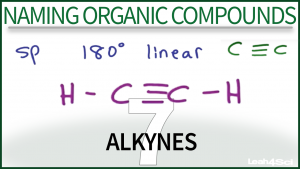
This tutorial video shows you how to identify and name organic compounds containing a carbon to carbon triple bond. Examples including naming simple alkynes and naming alkynes with substituents using my puzzle piece approach to IUPAC nomenclature.
(Watch on YouTube: Alkynes. Click CC on bottom right for transcript.)
<– Watch Previous Video: Naming Alkenes
–> Watch Next Video: Naming Enynes (alkene + alkyne on same compound)
This is Video 7 in the Naming Organic Compounds Video Series. Click HERE for the entire series.
Need a review on Functional Groups? Watch the Functional Groups Video, Download the Cheat Sheet, then try the Quiz.


