 Isoelectric point calculations are critical in your biochemistry course and MCAT prep. The isoelectric point or pI gives you the pH at which the molecule has a net zero charge. This comes from a balance of protonation and deprotonation so that all positive and negative charges cancel out.
Isoelectric point calculations are critical in your biochemistry course and MCAT prep. The isoelectric point or pI gives you the pH at which the molecule has a net zero charge. This comes from a balance of protonation and deprotonation so that all positive and negative charges cancel out.
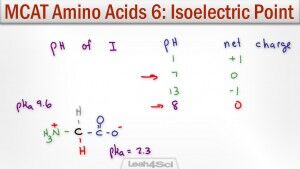
This video shows you how to calculate amino acid isoelectric point along with a shortcut to help you figure out WHICH pKa to use when given a side chain pKa value.
(Watch on YouTube: Isoelectric Point of Amino Acids. Click cc on the bottom right for video transcript)
< — Watch Previous Video: Zwitterion and Amino Acid Charge at Given pH value
— > Watch Next Video: Peptide Charge and Isoelectric Point Shortcut
This is video 6 in the Amino Acids Series. Click for complete series + Practice Quiz and Cheat Sheet
Have you grabbed the FREE Printable Cheat Sheet to follow along? CLICK HERE



Why is Tyrosine an acidic amino acid?
You said the rule for finding the PI for aa’s with proton-able side chains is: for an acidic aa (Asp, Glu, Cys, Tyr) use the pKa of the carboxy and the R-group, and for a basic aa (His, Lys, Arg) use the pKa of the amine and the R-group.
However in the previous example the pKa of the side chain of Tyr was above that of the amine and carboxy, and we did not use the pKa of that value to find the PI. Does this aa not follow the rule / Tyr is not an acidic aa?
Honestly, I am so grateful to you. It is absolutely amazing how well you go through these topics, and help students not rely on memorization but a deeper understanding of, and connection with the topics. Your videos are helpful and truly inspiring about education (for that, I thank you most). It’s very refreshing to be explained something this thoroughly and clearly. Awesome job! I hope your following grows, helping more people learn these concepts and learn from your pedagogy.
I’m reading Kaplan which has “MCAT Experties” saying that, “you are not expected to know the exact value for isoelectric points or side chain pKa values. The MCAT will provide you with numerical data to use in answering questions…”
Hi Leah! Do you think the pKa values of carboxylate group, side chain, and amine group will be given in the questions or do you happen to have a list of all the values for us to memorize? Thank you for this helpful video!
You are awesome! How do you figure the overall charge of a polypeptide when it contains amino acids that are polar neutral? I know that you need to use the pKa of the acids and bases of the polar, but what about neutral polar like S, M, W, N…? I know that C has a pKa to memorize, but most of the polar neutrals do not. So I’m wondering if you just draw an x when you are writing the pKas underneath the polypeptide like the alkyl nonpolar ones?
No one… I repeat no one can explain this like you! I have your voice in my head as I study these lol!
This is the most amazing video I’ve ever learned from. Some prep companies failed to explain this fully like you did, and I found myself making the mistakes you mentioned! This is great, wish I found it a bit earlier.
Rim, I’m glad this has been helpful to you! I was a student once too, so I know exactly what you mean. I try to provide the resources I wish I could have had. How long until you have your MCAT exam?