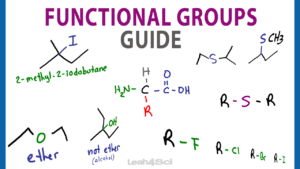
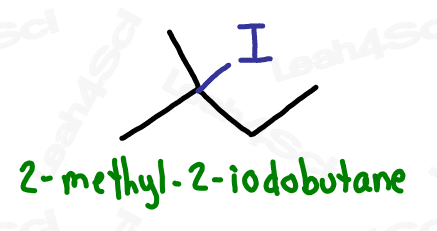Organic Chemistry Functional Groups: A Complete Guide to Recognizing, Drawing, and Naming (+ Priority Cheat Sheet at bottom)
A functional group is a specific group of atoms that helps determine the chemistry and reactivity of the overall molecule. When classifying functional groups, we look at both the specific atoms present, as well as the manner in which they are connected to each other.
While there are an overwhelming number of functional groups to consider, this guide will focus on the groups you’re most likely to come across at the beginner orgo level, along with some common groups that will show up in later (orgo 2) reactions.
We’ll tackle these groups from lowest to highest priority looking at
- Atomic makeup of each group
- How to recognize and draw them
- Key characteristics
- How to name each group.
This topic is also tested on the MCAT, GAMSAT, DAT and similar exams
Recall that if more than 1 functional group appears on a molecule, the higher priority group is named in the suffix, and the lower priority group is ‘demoted’ to substituent status and named in the prefix.
Follow along with the Functional Group Priority Cheat Sheet as well as any specific functional group naming videos that are linked after the examples below.
Here is a quick Functional Group overview before we jump into detail
Ready to Break Down The Individual Functional Groups?
Let's get started!!
The R Group
While the R group is not a functional group at all, it’s important to discuss it before we move on.
The letter ‘R’ pops up often in chemistry.
R represents a variable group, a changeable set of atoms that we don’t care to elaborate on at this moment.
For example, if you’ve studied the 20 common amino acids you’ll notice that the common amino acid structure has a backbone and an ‘R’ on the central carbon. That ‘R’ represents the 20 different side chains.
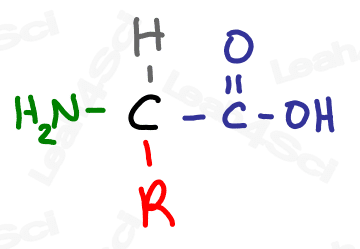 I like to think of the ‘R’ simply as the ‘REST’ of the molecule.
I like to think of the ‘R’ simply as the ‘REST’ of the molecule.
What does this have to do with functional groups?
Functional groups can show up on all sorts of carbon chains. But if the focus of our discussion is a functional group, we don’t want to get distracted by all of the carbons, hydrogens, or anything else in the parent chain.
Instead, we replace the entire parent chain with the letter R and attach the functional group to it.
For example, if I want to show you that OH is an alcohol, I will write that as R-OH.
R = rest of the molecule, OH = the group we’re looking at attached to the ‘rest’ of the molecule.
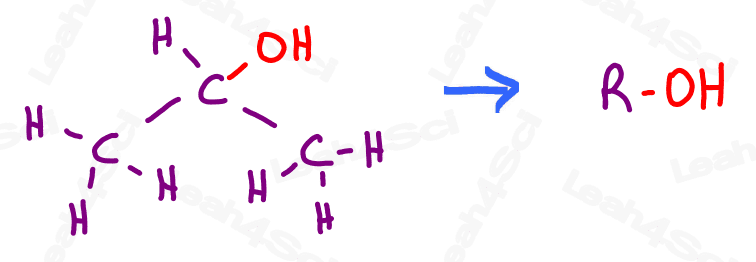 Ready? Let’s begin!
Ready? Let’s begin!
Alkyl Halide Substituent -X
The alkyl halide is considered a substituent rather than a functional group.
However, they are so reactive and show up so frequently in organic chemistry that we’d be remiss to skip over them.
While there are five halogens on the periodic table, I have yet to come across Astatine in over a decade of teaching Organic Chemistry.
Instead, focus only on Fluorine, Chlorine, Bromine and Iodine.
The letter ‘X’ acts as the ‘variable halogen’ and can represent any of the above.
Recall from the Intro to Orgo videos that electronegativity increases up and towards the right, while size increases down and towards the left.
This makes fluorine the smallest and most electronegative halogen, and Iodine the largest and least electronegative.
To recognize this group in organic molecules, simply look for a line to F, Cl, Br, or I
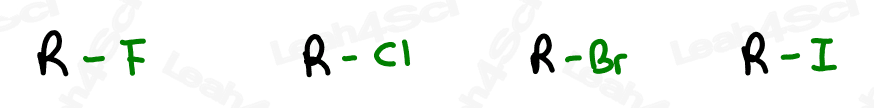
Naming Alkyl Halides
Since halogens are substituents rather than functional groups, we include their name in the prefix.
Take the full halogen’s name, drop the ending and add ‘o’.
For example, iodine becomes ‘iodo’, chlorine becomes ‘chloro’.
Be sure to specify the number of the carbon on which the halogen appears.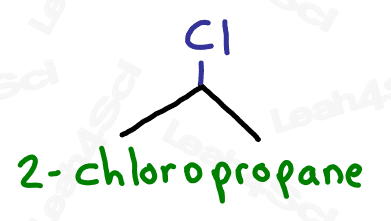
For even more watch: Naming Alkyl Halides
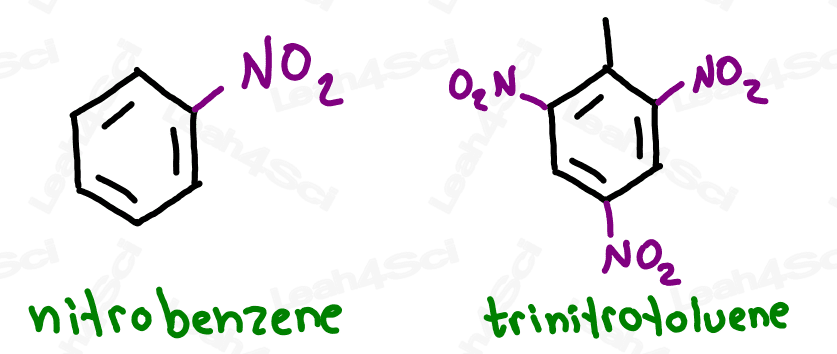
The Nitro Functional Group -NO2
While not required knowledge for naming and drawing early in organic chemistry, it is a tricky group that tends to confuse students when studying resonance and reactions.
Why?
The nitro group appears to be a very innocent neutral N + 2 O combination.
But if you draw it out, you’ll notice there’s much more going on.
A quick Lewis structure attempt reveals 2 major resonance forms with a positive formal charge on the nitrogen and a negative charge resonating between the 2 oxygen atoms.
The resonance hybrid has a partially positive nitrogen and 2 partially negative oxygens.
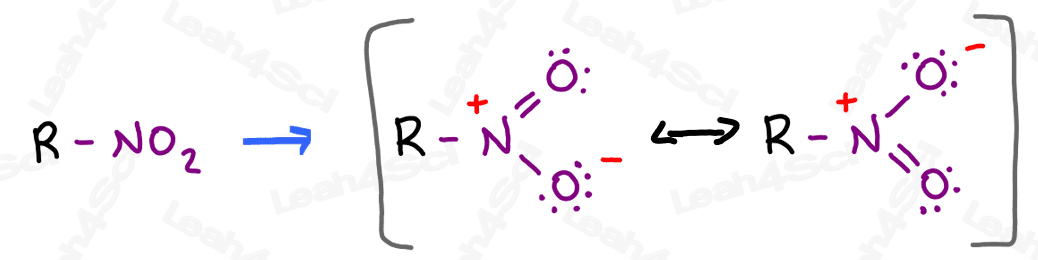
Naming Nitro Groups
You may not be tested on this, but do recognize that if NO2 appears on your molecule, you simply add the prefix ‘nitro’.
Thioether Functional Group -SR
Many students won’t cover thioethers in organic chemistry, and those who do typically see them in late orgo 1 reactions.
Anytime you see the prefix ‘thio’, immediately think of sulfur.
Thioether, as the name implies, is a thio (or sulfur) version of the ether.
As we’ll see shortly, ethers have an oxygen between 2 carbon atoms.
Thioethers have a sulfur (rather than oxygen) between 2 carbon atoms.

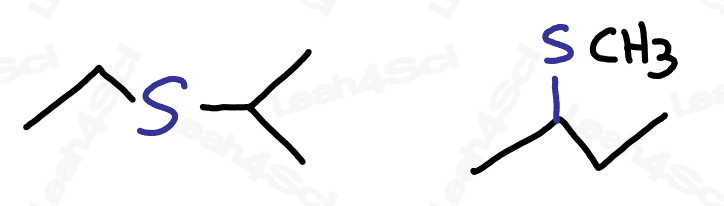
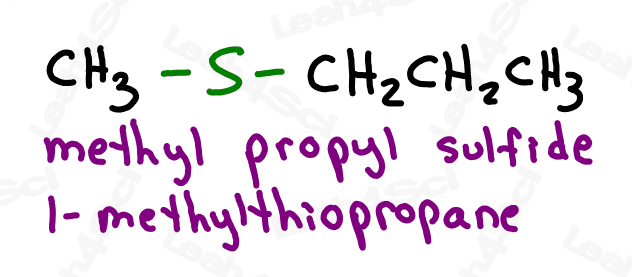
To recognize this functional group in organic molecules, look for a carbon chain with an S stuck in the middle, or look for a carbon chain with an S-R coming off it.
Naming Thioethers
Thioether groups are named with an ‘alkylthio’ prefix, where ‘alkyl‘ represents any R group. For a common name, list the alkyl group on either side of the sulfur in alphabetical order and end with the word ‘sulfide’.
Ether Functional Group -OR
Ethers are molecules that contain an oxygen atom sitting between 2 carbon atoms.
Not to be confused with an oxygen atom single bound to a branched carbon chain
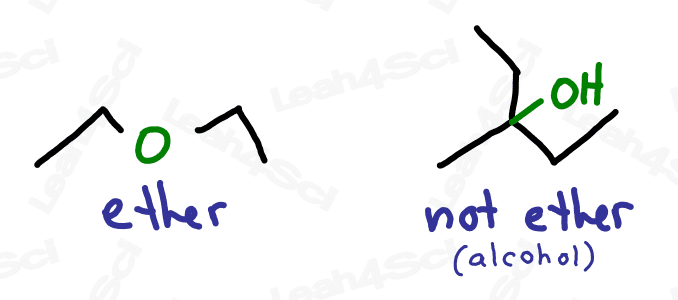 Think of this as an oxygen atom stuck into the molecule, breaking the carbon chains apart, rather than simply being attached to the parent chain as a substituent (though they’ll show up that way as well).
Think of this as an oxygen atom stuck into the molecule, breaking the carbon chains apart, rather than simply being attached to the parent chain as a substituent (though they’ll show up that way as well).
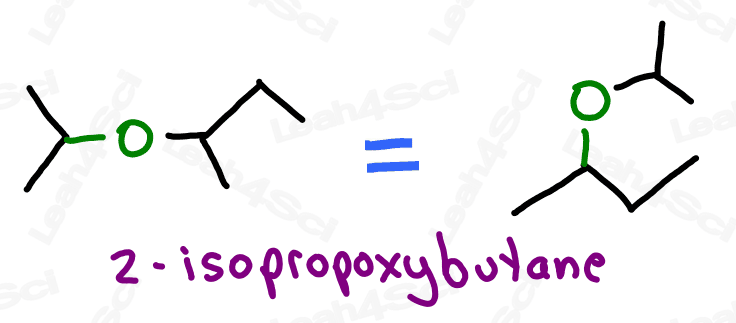 With oxygen bound to 2 carbon atoms, the ether is incapable of hydrogen bonding and exhibits overall lower polarity. This will come in handy when studying polar protic and aprotic solvents.
With oxygen bound to 2 carbon atoms, the ether is incapable of hydrogen bonding and exhibits overall lower polarity. This will come in handy when studying polar protic and aprotic solvents.
Naming Ethers
Like their sulfur counterparts, the ether group is an -OR group attached to the parent chain.
Ethers are named as ‘alkoxy’ groups: ‘alk’ for the shorter carbon chain, and ‘oxy’ for the oxygen atom.
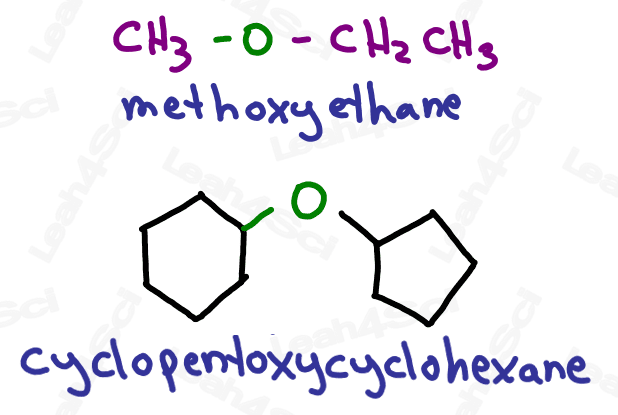 For even more watch: Naming Ethers
For even more watch: Naming Ethers
Amine Functional Group -NH2 -NHR -NR2 -NR3+
The amine is a functional group containing a nitrogen single bound to the parent chain. Whereas the nitrogen in the nitro functional group was attached to two oxygen atoms, the nitrogen in an amine may have 0-3 additional carbon groups attached to it.
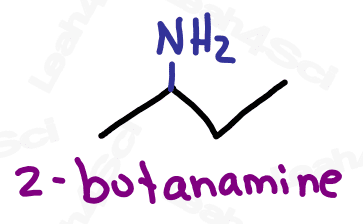
Amines are classified as primary, secondary, tertiary or quaternary based on how many TOTAL carbon atoms are attached to the nitrogen. This video goes into detail about a trick I use to identify the degree of substitution.
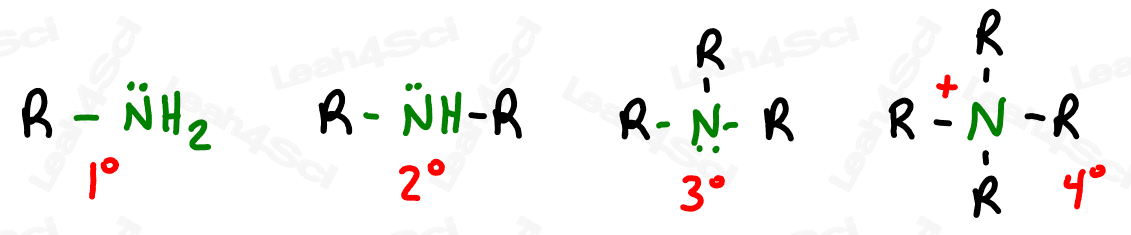 Primary, secondary and tertiary amines are neutral. Quaternary amines have a +1 formal charge, due to having 4 bonds and no lone pairs on the molecule.
Primary, secondary and tertiary amines are neutral. Quaternary amines have a +1 formal charge, due to having 4 bonds and no lone pairs on the molecule.
While only primary and secondary amines are capable of hydrogen bonding, all neutral amines are fairly reactive due to the partially negative lone pair on the nitrogen atom.
Aminium ions (positive quaternary amines) are also reactive but more likely to GET attacked due to their +1 charge.
Naming Amines
As the highest priority functional group, amines get the suffix -amine.
As a lower priority group, amines get the prefix ‘amino’.
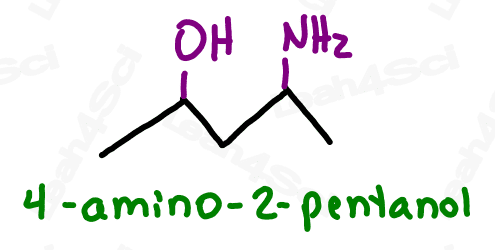
When naming the additional carbon groups on secondary and tertiary amines, name them as you would any carbon substituent (with a prefix added to the front of the name). Use the letter N to designate the position of the group, rather than a number on the carbon chain.
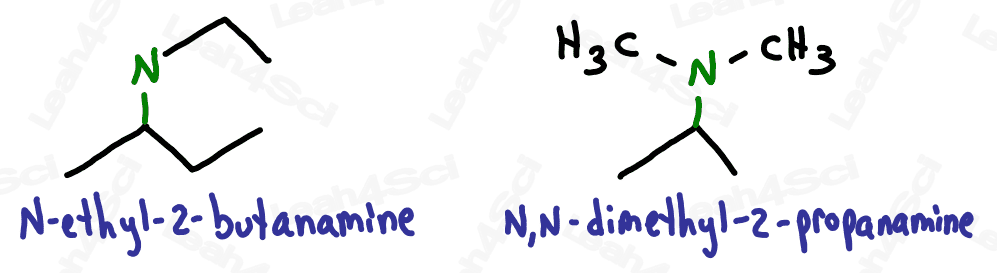 For even more watch: Naming Amines
For even more watch: Naming Amines
Thiol Functional Group -SH
We’ve already seen ‘thio’ denoting sulfur in the thioether.
The difference here is that thiol ends on ‘ol’ telling us it’s an alcohol.
The thiol group is a ‘thio’ alcohol, or sulfur alcohol.
Thio = sulfur and ol = alcohol.
 The sulfur atom is larger than oxygen making the thiol group less polar. Unlike alcohols, thiols are unable to participate in hydrogen bonding (due to their lower polarity).
The sulfur atom is larger than oxygen making the thiol group less polar. Unlike alcohols, thiols are unable to participate in hydrogen bonding (due to their lower polarity).
Naming Thiols
While you may not be tested on naming thiols, you will have to recognize them BY NAME when presented in a reaction (starting with substitution and elimination – SN/E reactions).
As the highest priority group, thiols get the suffix -thiol.

As a lower priority group, thiols get the prefix ‘mercapto’.
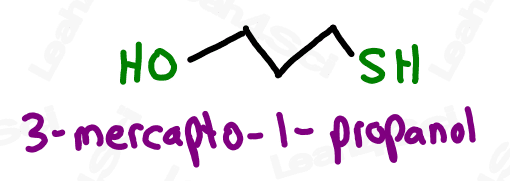
For even more watch: Naming Thiols
Alcohol Functional Group -OH
If alcohol makes you think of wine, beer or liquor, you’re not too far off. These beverages all contain one specific type of alcohol: ethanol.
However, the alcohol group refers to a wider range of compounds, anything containing an -OH functional group bound to the parent chain.
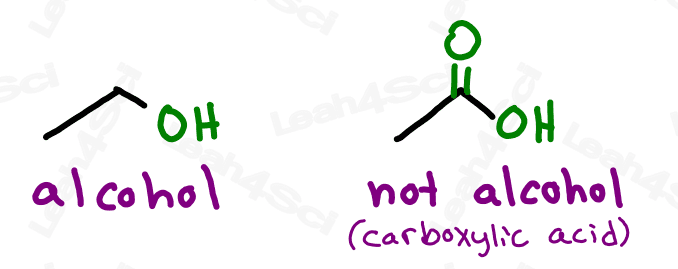
Or should I say JUST an OH bound to the parent. As we’ll see with carboxylic acids shortly, larger functional groups may contain OH as part of their overall structure.
 Oxygen is a very small and very electronegative atom. This makes oxygen-containing compounds polar, and makes the alcohol group, an oxygen bound to hydrogen, an ideal candidate for hydrogen bonding. This video has more on the physical properties of alcohols
Oxygen is a very small and very electronegative atom. This makes oxygen-containing compounds polar, and makes the alcohol group, an oxygen bound to hydrogen, an ideal candidate for hydrogen bonding. This video has more on the physical properties of alcohols
Naming Alcohols
As the highest priority group, alcohols get the suffix -ol.
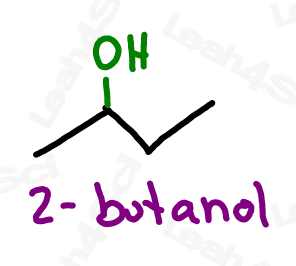
As a lower priority group, alcohols get the prefix ‘hydroxy’.
For even more watch: Naming Alcohols
The Carbonyl Group
While not a functional group itself, the carbonyl group is still worthy of our time given that it shows up in many of the upcoming functional groups. (You’ll see quite a few chapters dedicated to carbonyl reactions in Orgo 2)
The carbonyl group refers to a carbon double-bound to an oxygen atom. 
The carbonyl group is capable of resonance where the pi bond collapses up towards the oxygen.
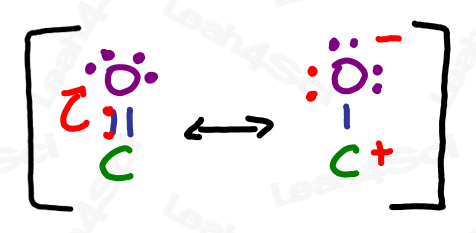
The charged resonance form is not very stable due to an incomplete octet on carbon, as well as the separation of charge between the positive carbon and negative oxygen.
So why do we care?
Recall from your resonance studies that molecules exist as a resonance hybrid, existing somewhere between contributing resonance forms. Therefore, the carbonyl resonance hybrid contains a partially positive carbon atom and partially negative oxygen. This partial separation of charge is crucial to so many upcoming orgo reactions.
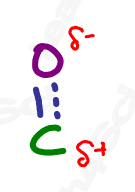
But, before we dive into all of the carbonyl reaction chapters, let’s take a look at all of the carbonyl containing functional groups. There’s a LOT of them.
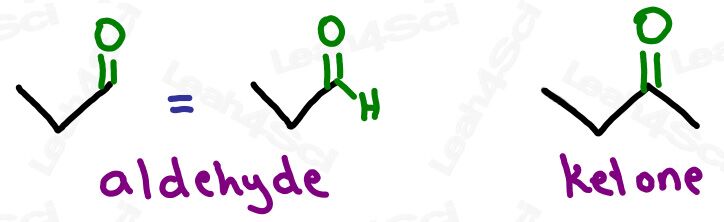
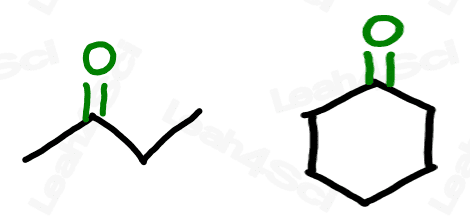
Ketone Functional Group -COR
The ketone functional group has a carbonyl sitting in the middle of a carbon chain. Think of this as a carbonyl bound to 2 R groups, or as an oxygen double-bound to a carbon somewhere in the middle of the chain.
‘Middle of the chain’ is your key identifier since the ketone is a carbonyl IN a molecule, rather than at the end of the molecule. (Those are coming soon).
My mnemonic for this: The ketONE doesn’t want to be alONE and so hides WITHIN the molecule.
Even though it’s pronounced ‘key’ + ‘tone’, it’s not spelled ‘keytone’. This is a fairly common mistake and one of my pet peeves.
Drawing Ketones
The ketone does not break up the carbon chain as we’ve seen with ethers or as we’ll see with the carboxylic acid derivatives like esters and amides. This allows us to draw a simple skeletal structure for the molecule, with 2 lines to oxygen at the ketone location.
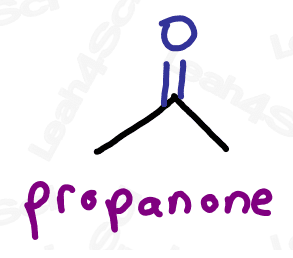
Naming Ketones
As the highest priority functional group, the ketone gets the suffix -one.
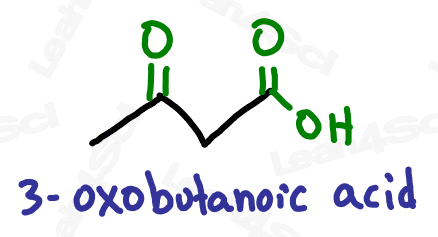
As a lower priority group, the ketone gets the prefix ‘oxo’.
For even more watch: Naming Ketones
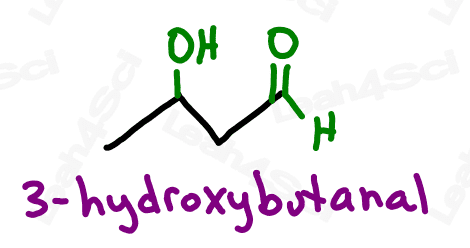
Aldehyde Functional Group -CHO
The aldehyde looks very similar to a ketone with one major difference:
Aldehydes are terminal functional groups.
Recognize the aldehyde as a carbonyl (C double-bound to O) at the end of the molecule, with a hydrogen rather than carbon (ketone) on the other side of the carbonyl.
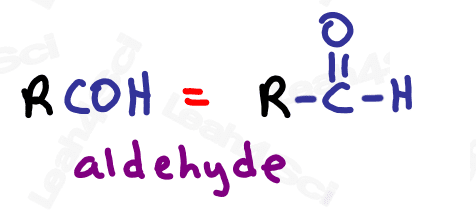
If you see a carbonyl at the end of the molecule with nothing else, it’s an aldehyde.
Drawing Aldehydes
Aldehydes are drawn just like ketones, but the carbonyl is at the end of the molecule.
While H atoms usually don’t have to be shown in skeletal structure, many will show the terminal H on the aldehyde to help emphasize its terminality. Expect to see them drawn both ways as I’ve drawn above.
Naming Aldehydes
As the highest priority functional group, aldehydes get the suffix -al.
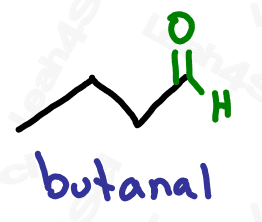
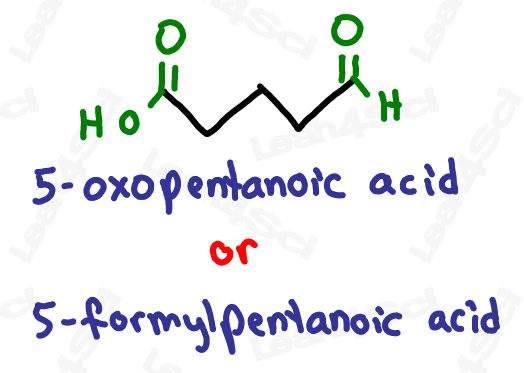
As a lower priority group, they get the prefix ‘oxo’ or ‘formyl’.
While both are correct, ‘oxo’ is more common.
For even more watch: Naming Aldehydes
But wait a minute.
If both ketone and aldehyde have the prefix ‘oxo’, how can we tell them apart?
How do we know if ‘oxo’ refers to an aldehyde or ketone?
Simple!
If the ‘oxo’ is on a terminal carbon, it must be an aldehyde which is a terminal group.
If the ‘oxo’ is on an internal carbon, then it must be a ketone.
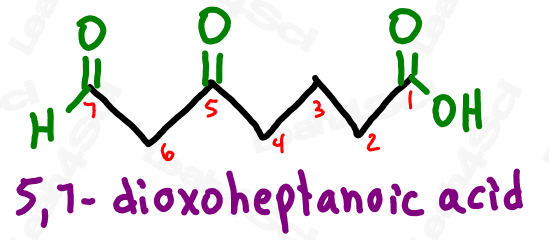
Take a look at the example below
Notice how ‘oxo’ on carbon 7 is the very last carbon in the chain? This must be an aldehyde.
Notice how ‘oxo’ on carbon 5 is NOT at the end of the chain? This must be a ketone.
Nitrile Functional Group -CN
Nitriles or cyano groups tend to show up in reactions more than naming for most orgo students.
The nitrile group is a carbon triple-bound to a nitrogen atom, where the carbon rather than the nitrogen is attached to the parent chain.
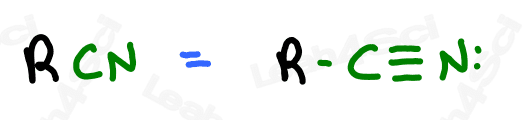
A carbon triple-bound to a nitrogen by itself rather than attached to a carbon chain is simply a cyanide anion.
For example KCN = Potassium Cyanide dissociates into a K+ cation and the CN- anion.
 The nitrile or cyano group will show up mostly in advanced orgo 2 reactions.
The nitrile or cyano group will show up mostly in advanced orgo 2 reactions.
Naming Nitriles or Cyano Groups
As the highest priority functional group, nitriles get the suffix nitrile.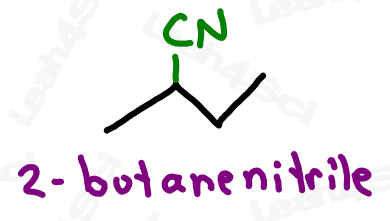
As a lower priority substituent, the nitrile gets a prefix of ‘cyano’.
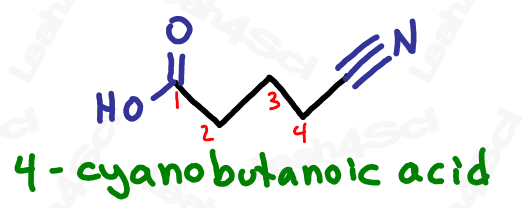
Due to the suffix ‘nitrile’ and prefix ‘cyano’, the name of the group itself is often interchangeable between ‘nitrile’ and ‘cyano’.
The sp2 carbon may or may not be counted as part of your parent chain. I’ve seen conflicting information regarding if to count it, or if to simply assume the C in CN is part of the substituent.
Be sure to find out which option your professor is looking for.
Carboxylic Acid Derivatives
For our final detour, let’s discuss carboxylic acid derivatives.
Since we’re working our way up from low to high priority, we’ll see the carboxylic acid last.
But let’s jump ahead for just a moment.
The carboxylic acid contains both a double-bound oxygen (carbonyl) and OH group on the same carbon. However, the carboxylic acid can undergo reactions where the OH is replaced with another group, as you’ll see toward the end of orgo 2.
Since these groups are derived FROM the carboxylic acid, they are called carboxylic acid derivatives.
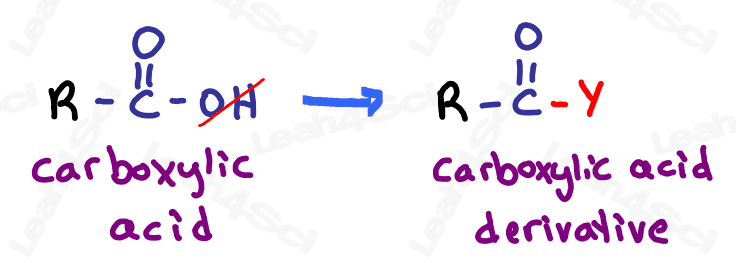 Let’s take a look at the 4 most common carboxylic acid derivatives that you’ll study in organic chemistry.
Let’s take a look at the 4 most common carboxylic acid derivatives that you’ll study in organic chemistry.
Amide Functional Group -CONH2 -CONRH -CONR2
The amide group is a carboxylic acid derivative where the OH is replaced by a nitrogen.
The amide is a terminal group with a carbonyl bound to nitrogen.
As with amines, the amide nitrogen can be bound to hydrogen, carbon, or both.
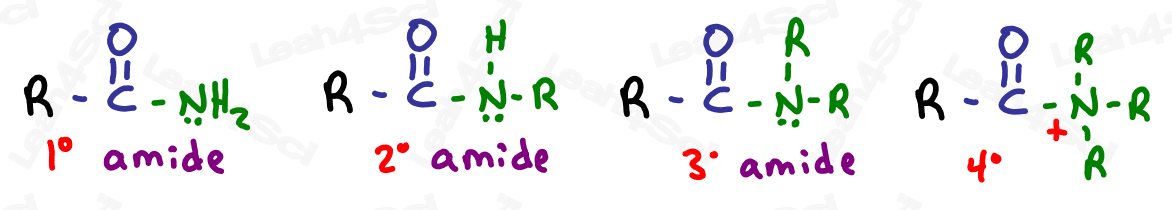
Drawing Amides
Amides are drawn like an aldehyde, with a terminal carbonyl. Instead of the H (aldehyde) add N, and then add H or C depending on the specific molecule in question.
Primary and secondary amides have highly polar bonds and are capable of Hydrogen Bonding making them soluble in water.
Tertiary amides are not capable of H-bonding due to not having any H atoms on the nitrogen.
Naming Amides
As the highest priority functional group, amides get the suffix -amide.
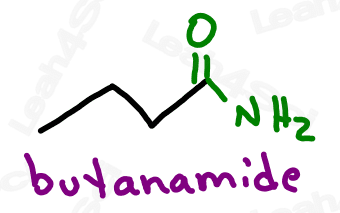
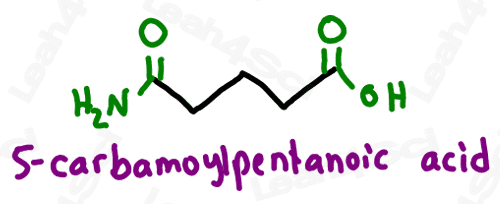
As a lower priority group (less likely to show up), amides get the prefix ‘carbamoyl’.
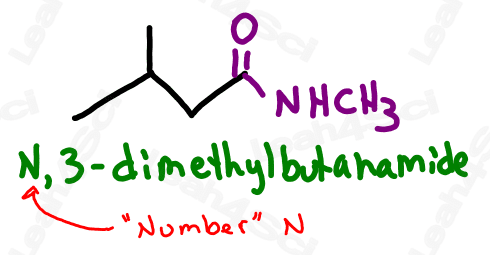
Amides containing alkyl substituents on the nitrogen are named just like amine substituents. Identify each substituent prefix, and use the letter N rather than a number to designate its location on the molecule.
The Acid Halide Functional Group -COX
The acid halide or acyl halide functional group comes from a carboxylic acid where the OH is replaced by a halogen or halide.
You’ll recognize the acid halide as a terminal group with a carbonyl bound to a halogen.
Acid halides are very unstable and highly reactive as you’ll see in your advanced orgo 2 reactions.

Drawing Acid Halides
Acid or acyl halides are drawn the same way as aldehydes but instead of an H at the end, you simply draw the specific halogen.
Naming Acid Halides
As the highest priority functional group, acid halides get the suffix -oyl halide (2 words) where the halide is replaced by the specific halogen present in the molecule.
For example ‘-oyl chloride’ or ‘-oyl bromide’.
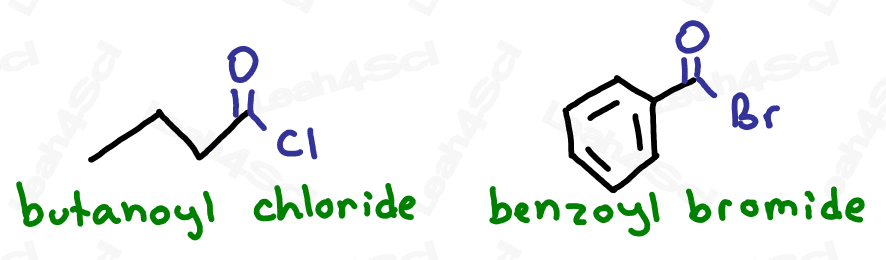
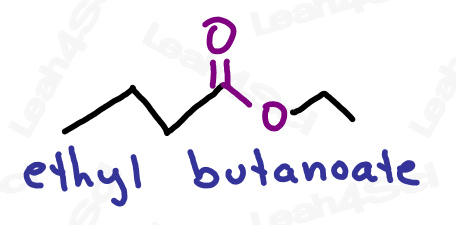
Ester Functional Group -COOR
The ester functional group has an oxygen double-bound to carbon (carbonyl) along with an OR group attached to the same carbon.
The ester is a carboxylic acid derivative in which the OH is replaced by an OR.
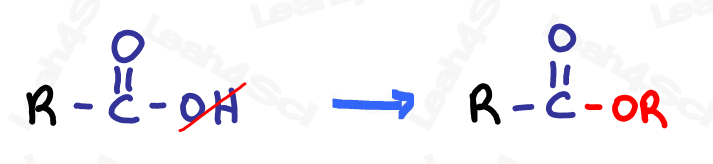
Do not confuse esters with ethers despite the names AND groups being so similar.
Ethers (OR) have just ONE oxygen between carbon atoms. Esters have a carbonyl in addition to the OR.

As a helpful memory device, one student pointed out on YouTube that Ester is a female name. From an anatomical point of view, females have 2 ‘O’s up top… if you know what I mean.
Naming Esters
As the highest priority functional group, esters get the suffix -oate.
The R-group on the ester is named as a substituent before the actual name (as a separate word, rather than connected)
For even more watch: Naming Esters
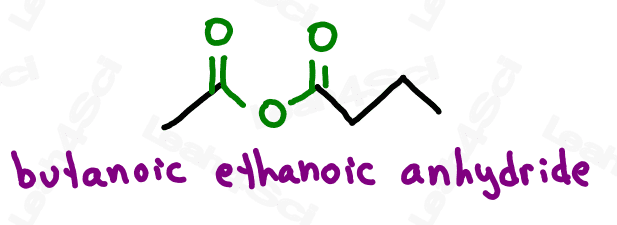
Acid Anhydride Functional Group
The acid anhydride, often simply called ‘anhydride’, is a somewhat tricky group. The anhydride contains a carbonyl, bound to oxygen, bound to another carbonyl.
This is less confusing when you consider that this carboxylic acid derivative is created by combining 2 carboxylic acids and removing a water molecule. (2H + O) in the process.
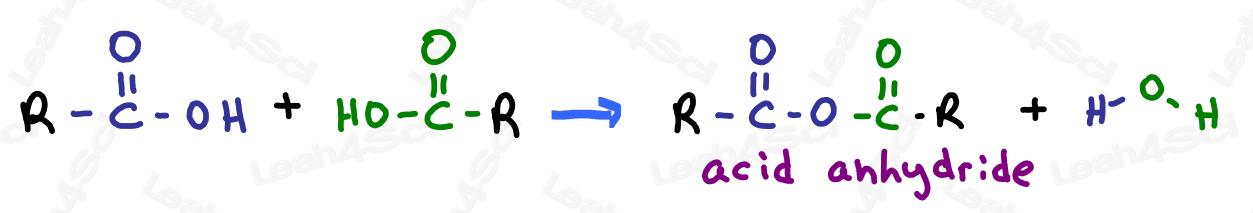
Anhydrides are very unstable and highly reactive, and will show up in late orgo 2 reactions.
Naming Anhydrides
You likely won’t have to name anhydrides but just in case:
Symmetrical anhydrides, which have the same length carbon chain on either side, are named similar to carboxylic acids with the ending -oic anhydride.
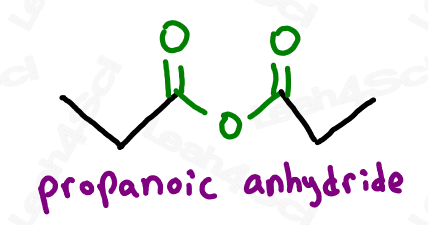
Assymetrical anhydrides, which have different length carbon chains on either side, are named for both carbon chains in alphabetical order, each ending in -oic followed by ‘anhydride’
Carboxylic Acid Functional Group -COOH
Finally we get to the highest priority functional group: the carboxylic acid.
Carboxylic acids have a carbon atom double bound to oxygen (carbonyl) along with an OH single bound to that same carbon atom.
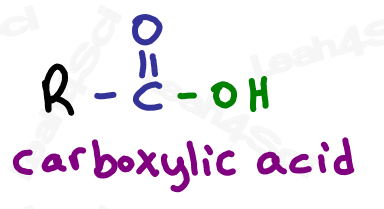
Do not confuse the carboxylic acid with an alcohol. If the OH is on a carbon that has nothing else, it’s an alcohol. If the OH is bound to a carbonyl carbon, it’s a carboxylic acid.
Carboxylic acids are fairly acidic due to hydrogen sitting on oxygen next to a carbonyl. When deprotonated, the resulting carboxylate anion is stabilized through resonance as shown below
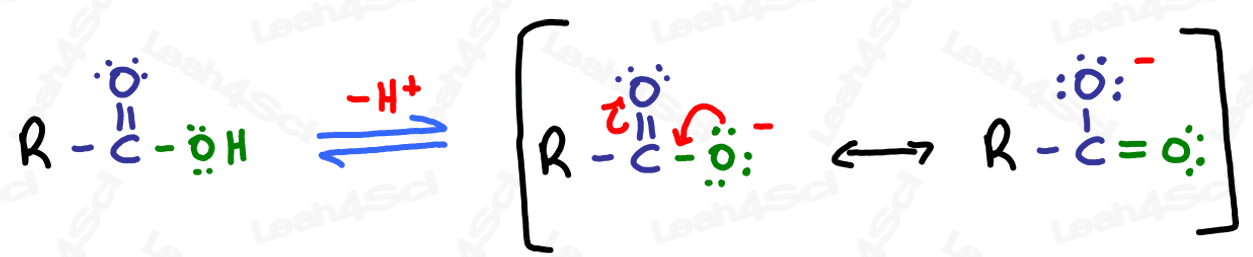
Naming Carboxylic Acids
As the highest priority functional group, carboxylic acids will always be the suffix of your molecule. Additionally, as a terminal functional group, always assume the carboxylic acid is located at carbon #1. This means no numerical designator is required.
Name the parent chain for the number of carbon atoms, then add the suffix -oic acid.
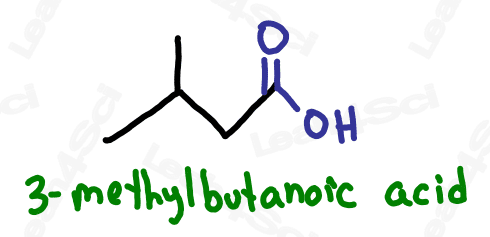
For even more watch: Naming Carboxylic Acids
Ready to Start Memorizing Organic Chemistry Functional Groups?
Grab yourself a Mini Whiteboard (<- Amazon referral link) and take an active approach using the Active Writing technique I share below:
What do you think?
Do you feel better about Organic Chemistry Functional Groups having read through the entire guide? Why not test your knowledge with the Functional Groups Practice Quiz?
Need a quick review? Watch the functional groups summary video, or take a look at the functional groups cheat sheet below.