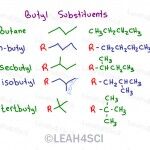
When you hear n-butyl, secbutyl, isobutyl and tert butyl. Do these terms scare you? Learning Organic Chemistry is like learning a new language where certain prefixes and suffixes will mean different things. Different ways of arranging bits and pieces will bring you to different variations of compounds. But just like a new language, the more you see it, […]
Organic Chemistry Tutorial Videos
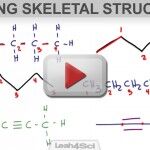
How to Draw Skeletal Structures of Organic Compounds
Organic molecules can become complicated very quickly. Skeletal structures give you a simple way to quickly represent these organic structures. Once you’ve watched the tutorial video below, make sure to work through my Skeletal Structures Practice Quiz to practice going from condensed formula to line structures and back again. This video will teach you how […]
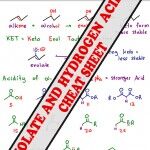
Enolate Formation and Alpha Hydrogen pKa Table
Alpha Hydrogen acidity comes from the ability to resonate negative conjugate electrons onto the nearby carbonyl group. Your professor may ask you to memorize pKa values. I recommend UNDERSTANDING this chart as explained in the enolate video so that you’re not left fumbling for numbers. <– Back to the Enolate Reactions Video Tutorial Series Homepage
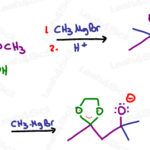
Grignard Reaction Mechanism Reagent and Cheat Sheet
The Grignard reaction (pronounced Grin-yard) involves an R-Mg-X, a carbon chain bound to a magnesium halide, typically used to form alcohols by attacking carbonyls such as in aldehydes or ketones. The Grignard reaction is my go-to for chain elongation in orgo 2 synthesis.Alkynes are my go-to for orgo 1 chain elongation. In English, please? Let’s back up […]
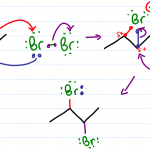
Acetal Reactions Practice Quiz
The quiz below requires some mastery in reactions, intermediates, and step-by-step mechanisms. Not ready? Start by gaining your foundation in the Acetal Tutorial Video Series! Scroll to the very bottom of this quiz for a link to the FREE quiz solutions PDF (Coming Soon!) to ensure you’ve got this down. The solutions walkthrough video is available in the […]
Halogenation Of Alkenes Tutorial Video
Alkene Reactions Series: Video 4 Halogenation of alkenes is the reaction in which a double bond is broken and replaced by a vicinal dihalide – 2 halogen atoms added to neighboring carbons. This reaction follows a pattern of anti addition. The goal of this video is to help you understand rather than memorize concepts related […]
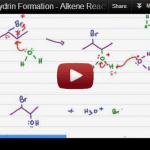
Halohydrin Formation
Alkene Reactions Series: Video 5 This tutorial takes you through the halohydrin formation mechanism, a type of electrophilic addition which adds both a halogen and alcohol to an alkene by using water instead of an inert solvent like CH2Cl2 or CCL4 to yield a halohydrin which features both a halogen and alcohol on the carbon […]
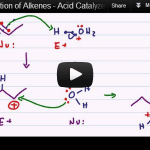
Acid Catalyzed Hydration of Alkenes
Acid catalyzed hydration of alkenes is the reaction in which a pi bond is broken and an alcohol added to the Markovnikov position when carried out in water. When alcohol is used as the reaction solvent, an ether will form following the same reaction mechanism. The video below is Part 6 in my Alkene Reaction […]
Hydride Shift and Methyl Shift Mechanism
Hydride shifts and methyl shifts can occur in organic chemistry reactions if there is a carbocation intermediate. While Markovnikov’s rule places the carbocation on the more substituted of the 2 former sp2 (double-bound) carbon atoms, if there is an EVEN MORE substituted carbon nearby, you’ll get a carbocation rearrangement. These videos help you understand the […]
Markovnikov vs Anti-Markovnikov in Alkene Addition Reactions
Markovnikov’s Rule tells us where to add the nucleophile and hydrogen in an asymmetrical alkene addition reaction. This is a critical pattern to both understand and recognize when studying alkene addition reactions. What is Markovnikov’s rule all about and how does this impact regioselectivity in electrophilic addition reactions? As you follow along with my alkene reactions […]
- 1
- 2
- 3
- …
- 15
- Next Page »