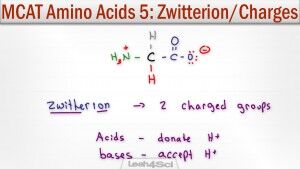
 Amino acids exist as zwitterions at physiological pH. A zwitterion by definition is a molecule with 2 (zwitter) ions, one positive and one negative for a net zero charge.
Amino acids exist as zwitterions at physiological pH. A zwitterion by definition is a molecule with 2 (zwitter) ions, one positive and one negative for a net zero charge.
This video shows you how to quickly calculate amino acid charge at any given pH by helping you recognize when a given side chain is protonated or deprotonated.
(Watch on YouTube: Zwitterion and Amino Acid Charge. Click cc on the bottom right for video transcript)
< — Watch Previous Video: Stereochemistry of Amino Acids RS to DL
— >Watch Next Video: Isoelectric Point of Amino Acids with MCAT shortcut
This is video 5 in the Amino Acids Series. Click for complete series + Practice Quiz and Cheat Sheet
Have you grabbed the FREE Printable Cheat Sheet to follow along? CLICK HERE


