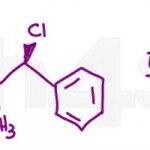
Substitution and Elimination reactions are potentially the most difficult concepts covered at the Organic Chemistry 1 level. In addition to studying the SN1 SN2 E1 and E2 reaction mechanisms, you also have to understand the similarities and differences so that you can derive the correct products for specific reaction conditions. I’ve put together this medium/tricky quiz […]
SN1 Reaction Mechanism Examples of Unimolecular Substitution Part 2
SN1 SN2 E1 Series: Video 16 Unimolecular Nucleophilic Substitution video 2 of 3 – this video takes you through additional SN1 reaction mechanism examples. As you work through this video pay attention to the logic, concepts, and especially patterns. Doing so will help you understand when to choose SN1 while avoiding confusing memorization (Watch on […]
Orgo Mechanisms with Common Arrow Patterns
Organic chemistry is all about mechanisms. And while the sheer volume may feel overwhelming, they all boil down to a few simple types of mechanism steps. In Part 1 and Part 2 we looked at the four common mechanism types: Nucleophilic attack, Loss of Leaving Group, Proton transfer and Rearrangements. In this video we’ll look at TWO […]
Nucleophilicity vs Basicity in Substitution Elimination Reactions Part 1
SN1 SN2 E1 Series: Video 4 As an orgo tutor, one of the most common substitution/elimination questions I get relates to “What’s the difference between a nucleophile or base?” or “How can I tell a nucleophile from a base?” And the answer? They are THE SAME! Watch this video to understand the concept of Nucleophile […]
Choosing Between SN1 and SN2 Reactions Part 1
SN1 SN2 E1 Series: Video 15 Knowing that your alkyl halide will undergo nucleophilic substitution is not enough. As you work through these reactions pay attention to the key factors that help you distinguish between unimolecular and bimolecular substitution reactions. This is SN1 vs SN2 video one. Additional examples in the next video (Watch on […]
SN2 Reaction vid Bimolecular Nucleophilic Substitution Part 3
SN1 SN2 E1 Series: Video 14 Bimolecular substitution is a fast reaction which requires a good leaving group. Or at least one that can be kicked out easily. However, when faced with a bad leaving group, you must first ‘bribe’ the atom turning into a more willing leaving group before proceeding with the reaction. This […]
SN2 Reaction Chirality and Mechanism of Bimolecular Substitution Part 2
SN1 SN2 E1 Series: Video 13 When starting with a chiral alkyl halide, the SN2 reaction will undergo a backside attack and thus an inversion in chirality. This video shows you a breakdown of the chiral inversion to help you understand how easily to identify chiral SN2 reaction products. (Watch on YouTube: SN2 Part 2. […]
SN1 Reaction Mechanism with Hydride Shift and Carbocation Rearrangement Part 3
SN1 SN2 E1 Series: Video 11 In this final SN1 video you’ll see tricky examples involving less substituted carbocation intermediates followed by carbocation rearrangements and hydride shifts. When working through these reactions pay special attention to the patterns that help determine the SN1 mechanism over a potential SN2 or E2 (Watch on YouTube: SN1 Part […]
SN2 Reaction Rate and Mechanism Bimolecular Substitution Part 1
SN1 SN2 E1 Series: Video 12 The first of 3 SN2 videos, this video gives you a detailed overview of the bimolecular nucleophilic substitution reaction, reaction rate, step by step mechanism. Pay special attention to the features that determine an SN2 reaction and the potential chirality of the final product. (Watch on YouTube: SN2 […]
Oxygen As A Leaving Group Using Tosylate And Mesylate in Substitution and Elimination Reactions
SN1 SN2 E1 Series: Video 8 Oxygen makes a poor leaving group in substitution and elimination reactions. That is, when left in its initial form. The key to kicking out an oxygen is to ‘bribe it’ by turning it into a better leaving group. Watch this video for a detailed explanation with examples using tosylate, […]